Chapter: 11th Physics : UNIT 9 : Kinetic Theory of Gases
Some elementary deductions from kinetic theory of gases
Some
elementary deductions
from kinetic theory of gases
From
equation (9.12), we know that PV=2/3
U But the internal energy of an ideal gas is equal to N times the average
kinetic energy (∈) of each molecule.
U = N∈
For
a fixed temperature, the average translational kinetic energy ∈ will remain constant. It implies that
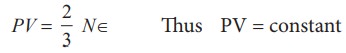
Therefore,
pressure of a given gas is inversely proportional to its volume provided the
temperature remains constant. This is Boyle’s law.
Charles’ law:
From
the equation (9.12), we get PV = 2/3U
For a fixed pressure, the volume of
the gas is proportional to internal energy of the gas or average kinetic energy
of the gas and the average kinetic energy is directly proportional to absolute
temperature. It implies that
V∝T
or V/T = constant
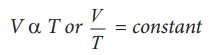
This
is Charles’ law.
Avogadro’s law:
This
law states that at constant temperature and pressure, equal volumes of all
gases contain the same number of molecules. For two different gases at the same
temperature and pressure, according to kinetic theory of gases,
From
equation (9.6)
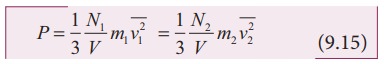
are
the mean square speed for two gases and N1 and N2 are the
number of gas molecules in two different gases.
At
the same temperature, average kinetic energy per molecule is the same for two
gases.

Dividing
the equation (9.15) by (9.16) we get N1
= N2
This
is Avogadro’s law. It is sometimes referred to as Avogadro’s hypothesis or
Avogadro’s Principle.
Related Topics