Kinetic Theory of Gases - Root mean square speed | 11th Physics : UNIT 9 : Kinetic Theory of Gases
Chapter: 11th Physics : UNIT 9 : Kinetic Theory of Gases
Root mean square speed
Root
mean square speed (vrms)
Root
mean square speed (vrms)
is defined as the square root of the mean of the square of speeds of all
molecules. It is denoted by vrms = √v2
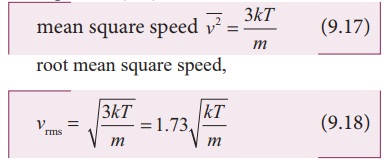
Equation
(9.8) can be re-written as,
From
the equation (9.18) we infer the following
(i) rms speed is directly proportional to square root of the temperature and inversely proportional to square root of mass of the molecule. At a given temperature the molecules of lighter mass move faster on an average than the molecules with heavier masses.
Example: Lighter molecules like hydrogen and helium have
high ‘vrms’ than heavier
molecules such as oxygen and nitrogen at the same temperature.
(ii)
Increasing the temperature will increase the r.m.s speed of molecules
We
can also write the vrms in
terms of gas constant R. Equation (9.18) can be rewritten as follows
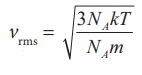
Where
NA is Avogadro number.
Since
NAk = R and NAm = M (molar mass)
The
root mean square speed or r.m.s speed

The
equation (9.6) can also be written in terms of rms speed
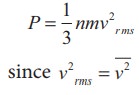
Impact
of vrms in nature:
1. Moon has no atmosphere.
The
escape speed of gases on the surface of Moon is much less than the root mean
square speeds of gases due to low gravity. Due to this all the gases escape
from the surface of the Moon.
2. No hydrogen in Earth’s atmosphere.
As
the root mean square speed of hydrogen is much less than that of nitrogen, it
easily escapes from the earth’s atmosphere.
In
fact, the presence of nonreactive nitrogen instead of highly combustible
hydrogen deters many disastrous consequences.
EXAMPLE 9.2
A
room contains oxygen and hydrogen molecules in the ratio 3:1. The temperature
of the room is 27°C. The molar mass of 02 is 32 g mol-1 and for H2 2 g mol-1. The
value of gas constant R is 8.32 J mol-1K-1
Calculate
(a)
rms speed of oxygen and hydrogen molecule
(b)
Average kinetic energy per oxygen molecule and per hydrogen molecule
(c)
Ratio of average kinetic energy of oxygen molecules and hydrogen molecules
Solution
(a)
Absolute Temperature
T=27°C
=27+273=300 K.
Gas
constant R=8.32 J mol-1k-1
For
Oxygen molecule: Molar mass
M=32
gm=32 x 10-3 kg mol-1
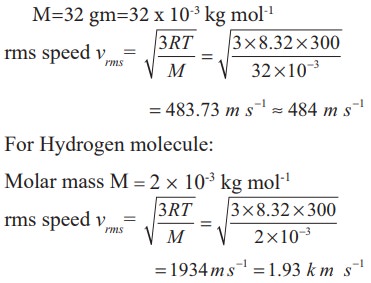
Note
that the rms speed is inversely proportional to √M and the molar mass of oxygen
is 16 times higher than molar mass of hydrogen. It implies that the rms speed
of hydrogen is 4 times greater than rms speed of oxygen at the same
temperature.
1934/484
≈ 4 .
(b)
The average kinetic
energy per molecule is 3/2 kT. It
depends only on absolute temperature of the gas and is independent of the
nature of molecules. Since both the gas molecules are at the same temperature,
they have the same average kinetic energy per molecule. k is Boltzmaan constant.

(c)
Average kinetic energy
of total oxygen molecules = 3/2 N0kT where N0 - number of
oxygen molecules in the room
Average kinetic
energy of total hydrogen molecules = 3/2 NHkT where NH
- number of hydrogen molecules in the room.
It
is given that the number of oxygen molecules is 3 times more than number of
hydrogen molecules in the room. So the ratio of average kinetic energy of
oxygen molecules with average kinetic energy of hydrogen molecules is 3:1
Related Topics