Chapter: 11th Physics : UNIT 9 : Kinetic Theory of Gases
Expression for pressure exerted by a gas
PRESSURE
EXERTED BY A GAS
Expression
for pressure exerted by a gas
Consider
a monatomic gas of N molecules each having a mass m inside a cubical container
of side l as shown in the Figure 9.1
(a).
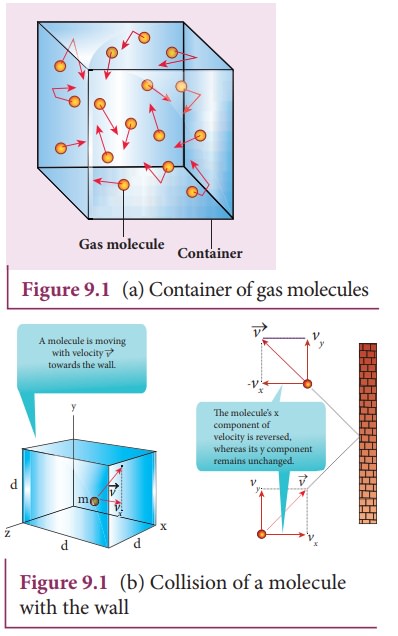
The
molecules of the gas are in random motion. They collide with each other and
also with the walls of the container. As the collisions are elastic in nature,
there is no loss of energy, but a change in momentum occurs.
The
molecules of the gas exert pressure on the walls of the container due to
collision on it. During each collision, the molecules impart certain momentum
to the wall. Due to transfer of momentum, the walls experience a continuous
force. The force experienced per unit area of the walls of the container
determines the pressure exerted by the gas. It is essential to determine the
total momentum transferred by the molecules in a short interval of time.
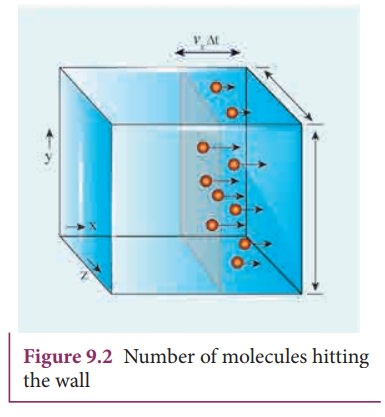
A
molecule of mass m moving with a velocity ![]() having components (vx,
vy, vz) hits the right side wall. Since we have assumed that the collision is elastic,
the particle rebounds with same speed and its x-component is reversed. This is
shown in the Figure 9.1 (b). The components of velocity of the molecule after
collision are (—vx, vy, vz).
having components (vx,
vy, vz) hits the right side wall. Since we have assumed that the collision is elastic,
the particle rebounds with same speed and its x-component is reversed. This is
shown in the Figure 9.1 (b). The components of velocity of the molecule after
collision are (—vx, vy, vz).
The x-component of momentum of the molecule before collision = mvx
The x-component of momentum of the molecule after collision = −mvx
The
change in momentum of the molecule in x direction
=Final
momentum – initial momentum = −mvx
−mvx = −2mvx
According
to law of conservation of linear momentum, the change in momentum of the wall =
2mvx
The
number of molecules hitting the right side wall in a small interval of time ∆t is calculated as follows.
The
molecules within the distance of vx∆t from the right side wall and moving
towards the right will hit the wall in the time interval ∆t. This is shown in the Figure 9.2. The number of molecules that
will hit the right side wall in a time interval ∆t is equal to the product of volume (Avx∆t) and
number density of the molecules (n).
Here A is area of the wall and n is number of molecules per unit volume
(N/V). We have assumed that the number density is the same throughout the cube.
Not
all the n molecules will move to the right, therefore on an average only half
of the n molecules move to the right and the other half moves towards left
side.
The
number of molecules that hit the right side wall in a time interval

In
the same interval of time ∆t, the
total momentum transferred by the molecules
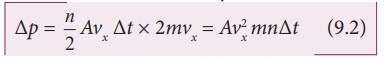
From
Newton’s second law, the change in momentum in a small interval of time gives
rise to force.
The
force exerted by the molecules on the wall (in magnitude)

Pressure,
P = force divided by the area of the wall

Since
all the molecules are moving completely in random manner, they do not have same
speed. So we can replace the term vx2 by the average ![]() x2 in
equation (9.4)
x2 in
equation (9.4)

Since
the gas is assumed to move in random direction, it has no preferred direction
of motion (the effect of gravity on the molecules is neglected). It implies
that the molecule has same average speed in all the three direction. So, 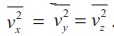 . The mean square speed is written as
. The mean square speed is written as
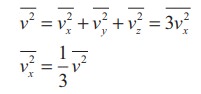
Using
this in equation (9.5), we get
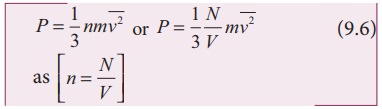
The
following inference can be made from the above equation. The pressure exerted
by the molecules depends on
(i) Number density n
= N/V. It implies that if
the number density increases then pressure will increase. For example when we
pump air inside the cycle tyre or car tyre essentially the number density
increases and as a result the pressure increases.
(ii) Mass of the molecule Since the pressure arises due to momentum transfer to the
wall, larger mass will have larger momentum for a fixed speed. As a result the
pressure will increase.
(iii) Mean square speed For a fixed mass if we increase the speed, the average speed will also increase. As
a result the pressure will increase.
For
simplicity the cubical container is taken into consideration. The above result
is true for any shape of the container as the area A does not appear in the
final expression (9.6). Hence the pressure exerted by gas molecules on the wall
is independent of area of the wall.
Related Topics