Chapter: 11th Physics : UNIT 9 : Kinetic Theory of Gases
Relation between pressure and mean kinetic energy
Relation
between pressure
and mean kinetic energy
From
earlier section, the internal energy of the gas is given by
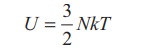
The
above equation can also be written as
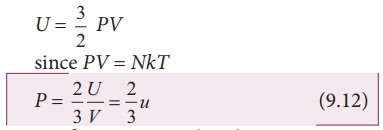
From
the equation (9.12), we can state that the pressure of the gas is equal to two
thirds of internal energy per unit volume or internal energy density (u=U/V).
Writing
pressure in terms of mean kinetic energy density using equation (9.6)
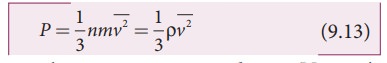
where
ρ = nm = mass density (Note n is number density)
Multiply
and divide R.H.S of equation (9.13) by 2, we get

From
the equation (9.14), pressure is equal to 2/3 of mean kinetic energy per unit
volume.
Related Topics