Chapter: Modern Analytical Chemistry: Spectroscopic Methods of Analysis
Quantitative Applications Using Molecular Luminescence
Quantitative
Applications Using Molecular Luminescence
Molecular fluorescence and,
to a lesser extent, phosphorescence have been used
for the direct or indirect quantitative analysis of analytes
in a variety of matrices. A di- rect quantitative analysis is feasible
when the analyte’s quantum yield for fluores-
cence or phosphorescence is favorable. When the analyte
is not fluorescent or phos- phorescent or when the
quantum yield for
fluorescence or phosphorescence is unfavorable, an indirect
analysis may be feasible. One approach
to an indirect analysis is to react
the analyte with
a reagent, forming
a product with
fluorescent properties. Another approach
is to measure a decrease
in fluorescence when the an- alyte is added to a solution
containing a fluorescent molecule. A decrease
in fluores- cence is observed when the reaction
between the analyte
and the fluorescent species enhances radiationless deactivation, or produces a nonfluorescent product. The ap- plication of fluorescence and phosphorescence to inorganic and organic analytes
is considered in this section.
|
2 |
Inorganic Analytes
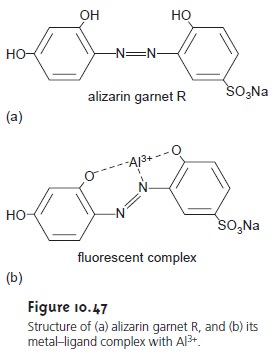
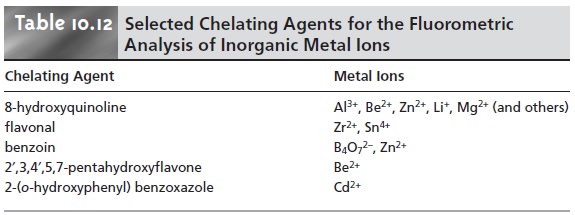
Organic Analytes
As noted earlier, organic compounds containing aromatic rings generally are fluorescent, but aromatic heterocycles are often phosphores cent. Many important biochemical, pharmaceutical, and environmental com- pounds are aromatic and, therefore, can be analyzed quantitatively by fluorometry or phosphorometry.
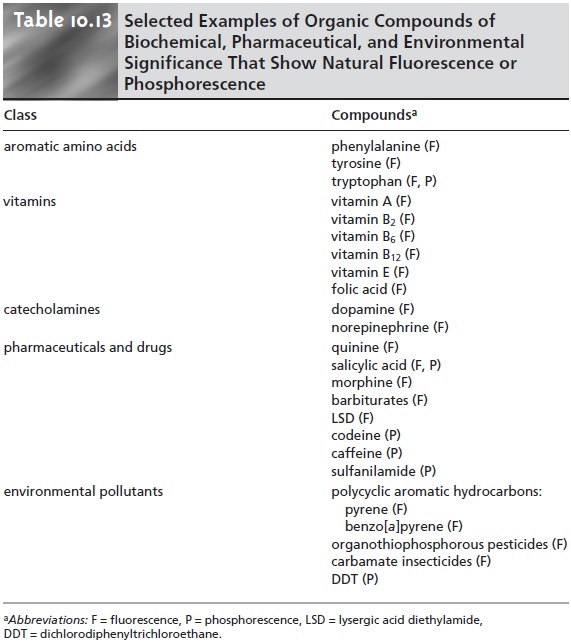
Several examples are
listed in Table
10.13. When an organic
ana- lyte is not naturally fluorescent or phosphorescent, it may be possible
to incorporate it into
a chemical reaction
that produces a fluorescent or phosphorescent product. For
example, the enzyme creatine phosphokinase can
be determined by using it to catalyze
the formation of creatine from phosphocreatine. The creatine that is formed reacts with ninhydrin, producing a fluorescent product of unknown
structure.
Standardizing the Method
Equations 10.32 and 10.33 show that the intensity of fluorescent or phosphorescent emission is proportional to the concentration of the photoluminescent species, provided that the absorbance of radiation from the exci- tation source (A = εbC) is less than approximately 0.01. Quantitative methods are usually standardized using a set of external standards. Calibration curves are linear over as much as four to six orders of magnitude for fluorescence and two to four orders of magnitude for phosphorescence. Calibration curves become nonlinear for high concentrations of the photoluminescent species at which the intensity of emis- sion is given by equation 10.31. Nonlinearity also may be observed at low concen- trations due to the presence of fluorescent or phosphorescent contaminants. As discussed earlier, the quantum efficiency for emission is sensitive to temperature and sample matrix, both of which must be controlled if external standards are to be used. In addition, emission intensity depends on the molar absorptivity of the pho- toluminescent species, which is sensitive to the sample matrix.
Related Topics