Chapter: Modern Analytical Chemistry: Spectroscopic Methods of Analysis
Molecular Fluorescence and Phosphorescence Spectra - Molecular Photoluminescence Spectroscopy
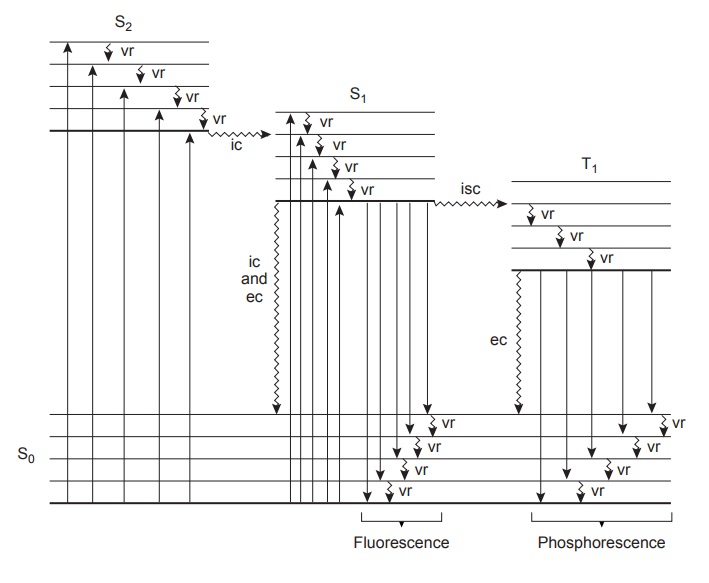
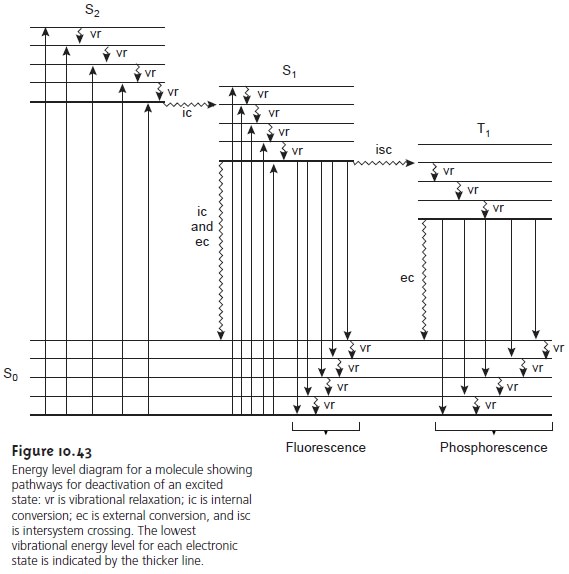
Molecular Fluorescence and Phosphorescence
Spectra
To appreciate the origin of molecular fluorescence and phosphorescence, we must
consider what happens to a molecule following the absorption of a photon.
Let’s as- sume that
the molecule initially occupies the lowest
vibrational energy level
of its electronic ground state. The ground state, which is shown in Figure 10.43, is a sin-
glet state labeled S0. Absorption of a photon of correct energy
excites the molecule to one of several
vibrational energy levels
in the first excited electronic state, S1, or the second electronic excited state, S2, both of which
are singlet states.
Relaxation to the ground
state from these
excited states occurs
by a number of mechanisms that are either radiationless, in that no photons are emitted, or involve the emission of a
photon. These relaxation mechanisms are shown in Figure 10.43. The most likely
pathway by which a molecule
relaxes back to its ground
state is that which gives
the shortest lifetime for the excited
state.
Radiationless Deactivation
One
form
of
radiationless deactivation is
vibra-
tional relaxation, in
which a molecule
in an excited vibrational energy
level loses energy as it moves
to a lower vibrational energy
level in the
same electronic state.
Vibrational relaxation is very rapid, with the molecule’s average
lifetime in an excited vibrational energy level
being 10–12 s or less. As a consequence, molecules that are excited
to different vibrational energy levels of the same excited elec- tronic state quickly return to the lowest vibrational energy level of this excited state.
Another form of radiationless relaxation is internal conversion, in which a
molecule in the ground vibrational level of an excited electronic state passes directly into a high vibrational energy level of a lower
energy electronic state
of the same spin state. By a combination of internal conversions and vibrational relaxations, a molecule in an excited electronic state may return
to the ground electronic state without emitting a photon.
A related form of radiationless relaxation is external
conversion in which
excess energy is transferred to the solvent
or another compo- nent in the sample matrix.
A final form of radiationless relaxation is an intersystem crossing in which
a molecule in the ground vibrational energy level of an excited
electronic state passes into a high vibrational energy level of a lower
energy electronic energy
state with a different spin state. For example, an intersystem crossing
is shown in Figure 10.43 between a singlet excited
state, S1, and a triplet excited
state, T1.
Fluorescence
Fluorescence occurs when a molecule in the lowest vibrational en- ergy level of an excited electronic state returns to a lower
energy electronic state
by emitting a photon.
Since molecules return
to their ground
state by the
fastest mech- anism, fluorescence is only observed
if it is a more efficient means
of relaxation than the combination of internal
conversion and vibrational relaxation. A quantitative expression of the efficiency of fluorescence is the fluorescent quantum yield, Φf, which is the fraction
of excited molecules returning to the ground state
by fluores- cence. Quantum
yields range from 1, when every molecule
in an excited state un- dergoes fluorescence, to 0 when fluorescence does not occur.
The intensity of fluorescence, If, is proportional to the amount of the radiation
from the excitation source that is absorbed
and the quantum
yield for fluorescence
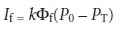 10. 29
10. 29
where k is a constant accounting for the efficiency of collecting and detecting the fluorescent emission. From Beer’s law we know that
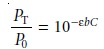 10.30
10.30
where C is the
concentration of the fluorescing species. Solving equation 10.30 for PT and
substituting into equation 10.29 gives, after simplifying
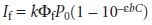 10.31
10.31
For low concentrations of the fluorescing species, where εbC is
less than 0.01,
this equation simplifies to
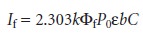 10.32
10.32
The intensity of fluorescence therefore, increases with an increase in quantum effi- ciency, incident power of the excitation source, and the molar absorptivity and con- centration of the fluorescing species.
Fluorescence is generally
observed with molecules
where the lowest energy ab- sorption is a π →
π* transition, although some
n
→ π* transitions show weak
fluo-rescence. Most unsubstituted, nonheterocyclic aromatic compounds show favorable
fluorescence quantum yields,
although substitution to the aromatic
ring can have a
significant effect on nf. For example,
the presence of an electron-withdrawing group, such as —NO2,
decreases Φ whereas adding an electron-donating group, such as —OH, increases
Φf. Fluorescence also increases for aromatic ring systems
and for aromatic molecules with
rigid planar structures.
A molecule’s fluorescence quantum yield is also influenced by external vari- ables such as temperature and solvent. Increasing temperature generally decreases Φf because more frequent
collisions between the molecule and the solvent
increases external conversion. Decreasing the solvent’s viscosity
decreases for similar
rea- sons. For an analyte with acidic or basic functional groups, a change
in pH may change the analyte’s structure and, therefore, its fluorescent properties. Changes in both the
wavelength and intensity of fluorescence may
be affected.
As shown in Figure 10.43, fluorescence may return the molecule to any of several vibrational energy levels in the ground
electronic state. Fluorescence, therefore, occurs over a range of wavelengths. Because
the change in energy for fluorescent
emission is generally less than that for absorption, a molecule’s fluorescence spec- trum is shifted
to higher wavelengths than its absorption spectrum.
Phosphorescence
A molecule in the lowest
vibrational energy level
of an excited triplet electronic state
normally relaxes to the ground
state by an intersystem cross- ing to a singlet
state or by external conversion. Phosphorescence is observed
when relaxation occurs by the emission
of a photon. As shown in Figure 10.43, phospho- rescence occurs over a range of wavelengths, all of which
are at a lower energy
than the molecule’s absorption band. The intensity of phosphorescence, Ip, is given by an
equation similar to equation 10.32 for fluorescence
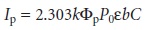 10. 33
10. 33
where Φp is the quantum yield for phosphorescence.
Phosphorescence is most favorable for molecules that have n →
π* transitions, which have a higher probability for an intersystem crossing than do π → π* transitions. For example, phosphorescence is observed with aromatic molecules contain- ing carbonyl groups
or heteroatoms. Aromatic
compounds containing halide
atoms also have a higher efficiency for phosphorescence. In general, an increase in phos-
phorescence corresponds to a decrease
in fluorescence.
Since the average
lifetime for phosphorescence is very long, ranging from 10–4 to 104 s, the quantum yield
for phosphorescence is usually quite
small. An improve- ment in Φp is realized by decreasing the efficiency of external conversion. This may be accomplished in several ways,
including lowering the temperature, using
a more viscous solvent, depositing the sample
on a solid substrate, or trapping the
molecule in solution.
Excitation Versus Emission Spectra
Photoluminescence spectra are recorded by
measuring the intensity of emitted
radiation as a function of either the excitation
wavelength or the emission wavelength. An excitation
spectrum is obtained
by monitoring emission at a fixed wavelength while varying the excitation wavelength. Figure 10.44 shows the excitation spectrum
for the hypothetical system described by the
energy level diagram
in Figure 10.43.
When corrected for
variations in source intensity and detector response, a sample’s excitation spectrum is nearly
identical to its absorbance spectrum. The excitation spectrum provides a convenient means
for selecting the best
excitation wavelength for
a quantitative or qualitative analysis.
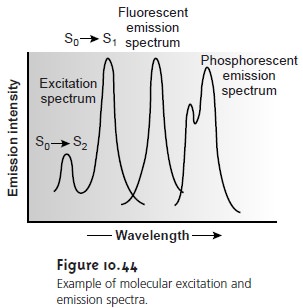
In an emission
spectrum a fixed wavelength is used to excite the mol-
ecules, and the intensity of emitted radiation
is monitored as a function
of wavelength. Although a molecule has only a single excitation spectrum, it has two
emission spectra, one for fluorescence and one for phosphores- cence. The corresponding emission
spectra for the hypothetical system in
Figure 10.43 are shown in Figure 10.44.
Related Topics