Chapter: Modern Analytical Chemistry: Spectroscopic Methods of Analysis
Quantitative Applications - Atomic Absorption Spectroscopy
Quantitative Applications
Atomic absorption using either flame or electrothermal atomization is widely used
for the analysis of trace
metals in a variety of sample matrices. Using the atomic
ab- sorption analysis for
zinc as an example, procedures have been developed for its de- termination in samples as diverse as water and
wastewater, air, blood,
urine, muscle tissue, hair,
milk, breakfast cereals, shampoos, alloys, industrial plating baths, gaso-
line, oil, sediments, and rocks.
Developing a quantitative atomic absorption method
requires several consider- ations, including choosing a method of atomization, selecting
the wavelength and slit
width, preparing the sample for analysis, minimizing spectral and chemical
in- terferences, and selecting a method of standardization. Each of these
topics is con- sidered in this section.
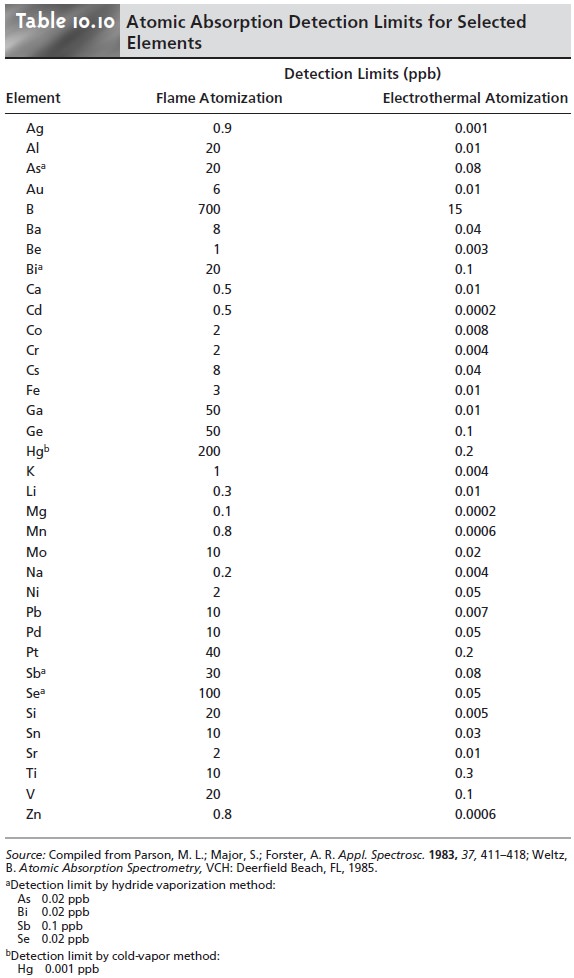
Flame Versus Electrothermal Atomization
The
choice of atomization method is determined primarily by the analyte’s concentration in the samples being
analyzed. Because of its greater sensitivity, detection limits for most elements
are significantly lower when using electrothermal atomization (Table 10.10).
A better precision when using flame atomization makes it the method of choice when the analyte’s concentration is significantly greater
than the detection limit for flame
atomization. In addition, flame
atomization is subject
to fewer interferences, allows for a greater
throughput of samples, and requires
less expertise from the operator. Electrother- mal atomization is the method of choice when the analyte’s concentration is lower than the
detection limit for flame atomization. Electrothermal atomization is also
useful when the volume of sample is limited.
Selecting the Wavelength and Slit Width
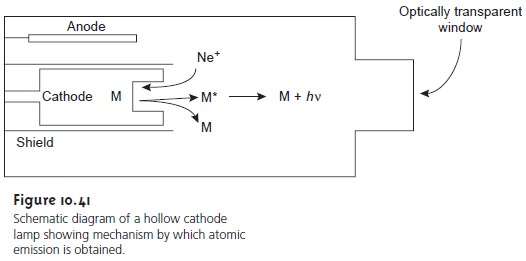
The source for atomic absorption is a hollow cathode
lamp consisting of a cathode
and anode enclosed
within a glass
tube filled with a low pressure
of Ne or Ar (Figure
10.41). When a potential is applied
across the electrodes, the filler
gas is ionized. The positively charged ions collide with the negatively charged
cathode, dislodging, or “sputtering,” atoms
from the cathode’s surface.
Some of the sputtered atoms
are in the excited state
and emit ra- diation characteristic of the metal
from which the cathode was manufactured. By
fashioning the cathode from the metallic analyte, a hollow cathode lamp provides emission lines that correspond to the analyte’s absorption spectrum.
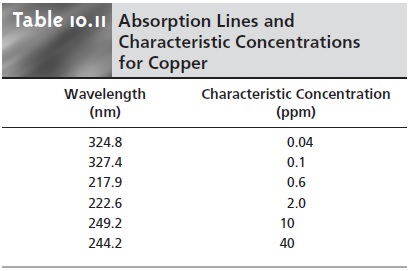
The sensitivity of an atomic
absorption line is often described by its characteris- tic concentration, which is the concentration of analyte giving
an absorbance of 0.00436 (corresponding to a percent transmittance of 99%). For example, Table
10.11 shows a list of wavelengths and
characteristic concentrations for
copper.
Usually the wavelength providing the best sensitivity is used, although a less sensitive wavelength may be more appropriate for a high concentration of analyte. A less sensitive wavelength also may be appropriate when significant interferences occur at the most sensitive wavelength. For example, atomizing a sample produces atoms of not only the analyte, but also of other components present in the sam- ple’s matrix. The presence of other atoms in the flame does not result in an inter- ference unless the absorbance lines for the analyte and the potential interferant are within approximately 0.01 nm. When this is a problem, an interference may be avoided by selecting another wavelength at which the analyte, but not the interfer- ant, absorbs.
The emission spectrum from a hollow
cathode lamp includes, besides emission
lines for the analyte, additional emission lines for impurities present
in the metallic cathode and the filler gas. These additional lines serve as a potential source of stray radiation that may lead to an instrumental deviation
from Beer’s law. Normally the monochromator’s slit width is set as wide as possible, improving
the throughput of radiation, while being narrow
enough to eliminate this source of stray radiation.
Preparing the Sample
Flame and electrothermal atomization require that the sample be in a liquid or solution form.
Samples in solid
form are prepared for analysis by dissolving in an appropriate solvent. When the sample is not soluble,
it may be digested,
either on a hot plate or by microwave, using HNO3, H2SO4, or HClO4. Alternatively, the analyte may be extracted
via a Soxhlet extraction. Liquid samples may be analyzed directly or may
be diluted or extracted if the matrix
is in- compatible with the
method of atomization. Serum samples, for instance, may be difficult to aspirate
when using flame
atomization and may produce unacceptably high background absorbances when using electrothermal
atomization. A liquid–liquid extraction using
an organic solvent
containing a chelating agent is frequently used to concentrate analytes. Dilute solutions of Cd2+, Co2+,
Cu2+, Fe3+, Pb2+,
Ni2+, and
Zn2+, for
example, can be concentrated by extracting with
a solu- tion of ammonium pyrrolidine dithiocarbamate in methyl
isobutyl ketone.
Minimizing Spectral Interference
A spectral interference occurs when an analyte’s absorption line
overlaps with an interferant’s absorption line or band.
As noted pre- viously, the overlap of two atomic absorption lines is seldom a problem.
On the other hand,
a molecule’s broad
absorption band or the scattering of source radia- tion is a potentially serious spectral interference.
An important
question to consider
when using a flame as an atomization source, is how to correct for the absorption of radiation by the flame. The prod- ucts of combustion consist
of molecular species
that may exhibit
broad-band ab- sorption, as well as particulate material that may scatter radiation from the source.
If this spectral interference is not corrected, then the intensity of the transmitted radiation decreases. The result
is an apparent increase in the sam- ple’s absorbance. Fortunately,
absorption and scattering of radiation by the flame are corrected by analyzing
a blank.
Spectral interferences also occur when components of the sample’s
matrix react in the flame
to form molecular species, such as oxides and hydroxides. Ab- sorption and scattering due to components in the sample
matrix other than the analyte constitute the sample’s background and may
present a significant prob- lem, particularly at wavelengths below
300 nm, at which the
scattering of radia-
tion becomes more important. If the composition of the sample’s matrix is known, then standards can be prepared
with an identical matrix. In this case the background
absorption is the same for both the samples and standards. Alterna- tively, if the background is due to a known
matrix component, then that compo-
nent can be added in excess to all samples
and standards so that the
contribution of the naturally occurring interferant is insignificant.
Finally, many interferences due to the
sample’s matrix can
be eliminated by adjusting the
flame’s composi- tion. For example, by switching to a higher
temperature flame it may be possible
to prevent the formation of interfering oxides
and hydroxides.
When the identity
of the matrix interference is unknown, or when it is impossi- ble to adjust the flame to eliminate the interference, then other means
must be used to
compensate for the background interference. Several methods have been devel- oped to compensate for
matrix interferences, and
most atomic absorption spec- trophotometers include one
or more of these methods.
One of the most common methods for background correction is the use of a continuum source, such as a D2 lamp. Since the D2 lamp is a continuum source, the absorbance of its radiation by the analyte’s narrow absorption line
is negligible. Any absorbance of radiation from the D2 lamp, therefore, is due to the
background. Absorbance of radiation from the hollow
cathode lamp, how- ever, is due to both the analyte and the background. Subtracting the absorbance for the D2 lamp
from that for the hollow
cathode lamp gives
an absorbance that has
been corrected for the background interference. Although this method of background
correction may
be quite effective,
it assumes that the background
absorbance is constant
over the range
of wavelengths passed
by the monochro- mator. When this is untrue, subtracting the two absorbances may under- or over-correct
for the background.
Other methods of background correction have been developed, including Zee- man effect background correction and Smith–Hieftje background correction, both of which are included
in some commercially available atomic absorption spec- trophotometers.
Minimizing Chemical Interferences
The quantitative analysis of some elements is complicated by chemical interferences occurring during
atomization. The two most common chemical
interferences are the formation of nonvolatile com- pounds containing the analyte and ionization of the analyte.
One example of a
chemical interference due to the formation of nonvolatile compounds is ob- served when PO43– or
Al3+ is
added to solutions
of Ca2+. In one study, for exam- ple, adding 100 ppm Al3+ to a solution
of 5 ppm Ca2+ decreased the calcium
ion’s absorbance from
0.50 to 0.14,
whereas adding 500
ppm PO43– to a similar solution of Ca2+ decreased the absorbance from 0.50 to 0.38.21 These interfer- ences were attributed to the formation
of refractory particles
of Ca3(PO4)2 and an
Al–Ca–O oxide.
The formation of nonvolatile compounds often can be minimized by increas-
ing the temperature of the
flame, either by changing the
fuel-to-oxidant ratio or by
switching to a different combination of fuel and oxidant. Another
approach is to add
a releasing agent
or protecting agent
to solutions containing the analyte. A releasing agent is a species whose
reaction with the interferent is more favorable than that of the analyte. Adding
Sr2+ or La3+ to solutions of Ca2+, for example,
minimizes the effect of PO43– and Al3+ by reacting
in place of the analyte.
Thus, adding 2000 ppm SrCl2 to the Ca2+/PO43– and Ca2+/Al3+ mixtures discussed in the preceding paragraph gave absorbances for each of 0.48, whereas
a solution of 2000 ppm SrCl2 and Ca2+ alone gave an absorbance of 0.49. Protecting agents
react with the analyte to form a stable volatile
complex. Adding 1% w/w EDTA to
the Ca2+/PO43– solution discussed in the preceding paragraph
gave an absorbance of 0.52, compared
with an absorbance of 0.55 for just the Ca2+ and EDTA. On the other hand, EDTA does not serve as a protecting agent for solutions
of Ca2+ and Al3+.
Ionization interferences occur
when thermal energy
from the flame
or elec- trothermal atomizer
is sufficient to ionize the analyte
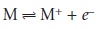 10.28
10.28
where M is the analyte
in atomic form,
and M+
is the cation
of the analyte
formed by ionization. Since
the absorption spectra
for M and M+ are different, the position of the equilibrium in reaction 10.28
affects absorbance at wavelengths where
M ab- sorbs. If another species
is present that ionizes more easily than M, then the equilib- rium in reaction 10.28
shifts to the left. Variations in the concentration of easily ionized species,
therefore, may have a significant effect on a sample’s absorbance, resulting in a determinate error. The effect of ionization can be minimized by adding a high concentration of an ionization suppressor, which is simply
another species that ionizes
more easily than the analyte.
If the concentration of the ioniza-
tion suppressor is sufficient, then the increased concentration of electrons in
the flame pushes reaction
10.28 to the left, preventing the analyte’s ionization. Potas- sium and cesium
are frequently used as ionization suppressors because of their low ionization energy.
Standardizing the Method
Because Beer’s law also applies to atomic absorp- tion, we might expect
atomic absorption calibration curves to be linear. In prac-
tice, however, most atomic absorption calibration curves are nonlinear, or linear for
only a limited
range of concentrations. Nonlinearity in atomic
absorption is a consequence of instrumental limitations, including stray radiation from the hol- low cathode lamp and a nonconstant molar absorptivity due to the narrow width of
the absorption line. Accurate
quantitative work, therefore, often requires a suitable means for computing the calibration curve
from a set
of standards. Non- linear calibration curves may be fit using quadratic and cubic equations, al- though neither
works well over
a broad range
of concentrations.
When possible, a quantitative analysis is best conducted using external stan- dards. Unfortunately, matrix interferences are a frequent problem, particularly when using electrothermal atomization. For this reason the method of standard additions is often used. One limitation to this method of standardization, however, is the re- quirement that there be a linear relationship between absorbance and concentration.
Related Topics