Chapter: Modern Analytical Chemistry: Spectroscopic Methods of Analysis
Overview of Spectroscopy
Overview of Spectroscopy
The focus of this is photon spectroscopy, using ultraviolet, visible,
and in- frared radiation. Because these techniques use a common
set of optical devices for dispersing and focusing the radiation, they often are identified as optical spectros- copies. For convenience we will usually
use the simpler term “spectroscopy” in place of photon spectroscopy or optical spectroscopy; however, it should be under- stood that we are
considering only a limited part
of a much broader area
of analyti- cal methods.
Before we examine
specific spectroscopic methods,
however, we first review the properties of electromagnetic radiation.
What Is Electromagnetic Radiation
Electromagnetic radiation, or light, is a form of energy whose behavior
is described by the properties of both waves
and particles. The optical properties of electromag- netic radiation, such as diffraction, are explained best by describing light as a wave.
Many of the interactions between
electromagnetic radiation and
matter, such as ab-
sorption and emission, however, are better described by treating light
as a particle, or photon. The exact nature
of electromagnetic radiation remains unclear, as it has since the development of quantum mechanics
in the first quarter of the twentieth century.3 Nevertheless, the
dual models of wave and
particle behavior provide
a use- ful description for electromagnetic radiation.
Wave Properties of Electromagnetic Radiation
Electromagnetic radiation consists of oscillating electric and magnetic fields that propagate
through space along a lin- ear
path and with a constant
velocity (Figure 10.1).
In a vacuum, electromagnetic
radiation travels at the speed
of light, c, which is 2.99792 x 108 m/s. Electromagnetic radiation moves through
a medium other
than a vacuum with a velocity, v, less
than that of the
speed of light
in a vacuum. The difference between v and c is
small enough (< 0.1%)
that the speed
of light to three significant figures, 3.00 x 108 m/s, is sufficiently accurate for most purposes.
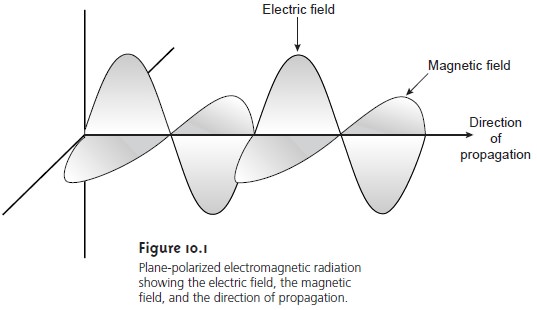
Oscillations in the electric and magnetic fields are perpendicular to each other, and to the direction of the wave’s propagation. Figure 10.1 shows an example of plane-polarized electromagnetic radiation consisting of an oscillating electric field and an oscillating magnetic field, each of which is constrained to a single plane. Normally, electromagnetic radiation is unpolarized, with oscillating electric and magnetic fields in all possible planes oriented perpendicular to the direction of propagation.
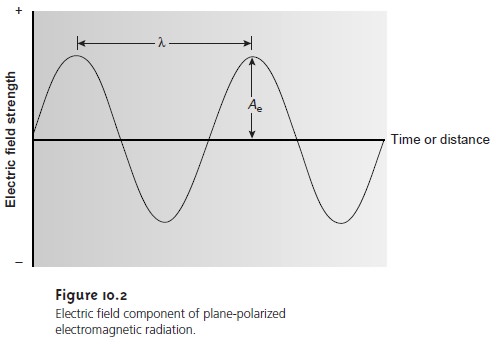
The
interaction of electromagnetic radiation with matter can
be explained using either the electric field
or the magnetic field. For this reason,
only the electric field component is shown
in Figure 10.2.
The oscillating electric
field is described by a sine wave of the form
E = Ae sin(2πvt + Φ)
where E is the magnitude of the electric
field at time t, Ae is the electric
field’s maxi- mum amplitude, v is the frequency,
or the number
of oscillations in the electric field per unit time,
and Φ is a phase
angle accounting for
the fact that
the electric field’s magnitude need not be zero at t = 0. An identical equation can be written for the magnetic field, M
M =
Am sin(2vt +
Φ)
where Am is the magnetic field’s maximum amplitude.
An electromagnetic wave, therefore, is characterized by several fundamental properties, including its velocity, amplitude, frequency, phase angle,
polarization, and direction of propagation. Other properties, which are based on
these funda- mental properties, also are useful for characterizing the wave
behavior of electro- magnetic radiation. The wavelength
of an electromagnetic wave, λ, is defined as the
distance between successive maxima, or successive minima (see Figure 10.2). For ultraviolet and visible electromagnetic radiation the wavelength is usually expressed in nanometers (nm, 10–9 m), and
the wavelength for
infrared radiation is given in microns (μm, 10–6 m). Unlike frequency, wavelength depends on the
electromag- netic wave’s velocity,
where
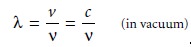
Thus, for electromagnetic radiation of frequency, v, the wavelength in vacuum is longer than in other
media. Another unit used to describe the wave properties of electromagnetic radiation
is the wavenumber, –v, which is the reciprocal of wave-
length
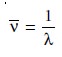
Wavenumbers are frequently used
to characterize infrared radiation, with the
units given in reciprocal centimeter (cm–1).
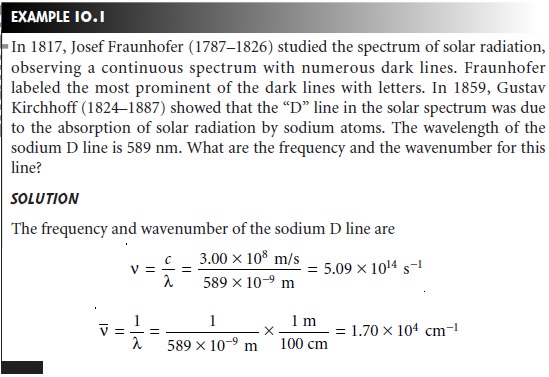
Two additional wave properties are power, P, and intensity, I, which give the flux of
energy from a source of electromagnetic radiation.
Particle Properties of Electromagnetic Radiation
When a sample absorbs electro- magnetic radiation it undergoes a change in energy. The
interaction between the sample and the electromagnetic radiation is easiest
to understand if we assume
that electromagnetic radiation consists of a beam of energetic particles
called photons. When a photon
is absorbed by a sample,
it is “destroyed,” and its
energy acquired by the
sample.5 The energy of a photon, in joules, is related to its frequency, wave- length, or wavenumber by the following equations
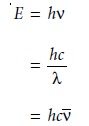
where h is Planck’s constant, which has a value of 6.626 x 10–34 J 1 s.
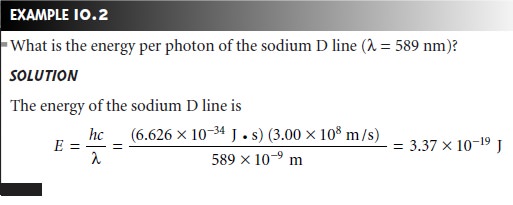
The energy of a photon
provides an additional characteristic property of electro-
magnetic radiation.
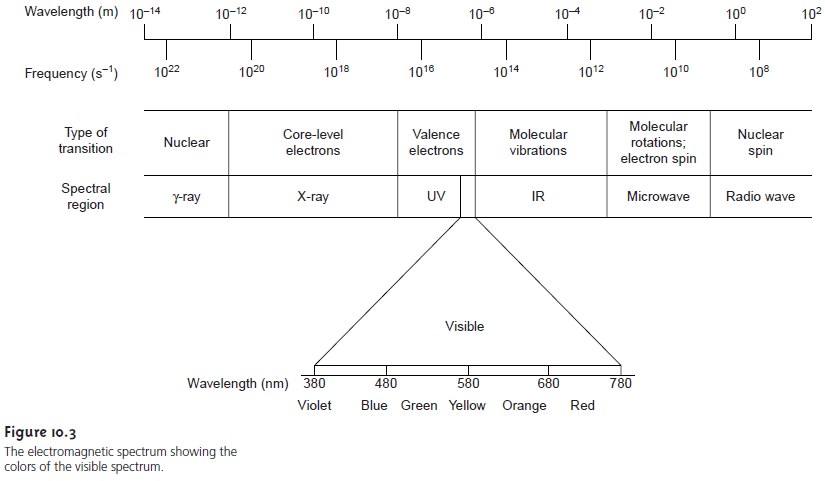
The Electromagnetic Spectrum
The frequency and wavelength of
electromagnetic radiation vary over many orders of magnitude. For
convenience, electromagnetic radiation is divided
into different regions
based on the
type of atomic
or molecular transition that gives rise to the absorption or emission of photons (Figure
10.3). The boundaries describing the electromagnetic spectrum are not rigid, and an
overlap between spectral
regions is possible.
Measuring Photons as a Signal
In the previous
section we defined
several characteristic properties of electromag- netic
radiation, including its energy, velocity, amplitude, frequency, phase angle,
polarization, and direction
of propagation. Spectroscopy is possible only if the pho-
ton’s interaction with the sample
leads to a change in one or more of these charac- teristic properties.
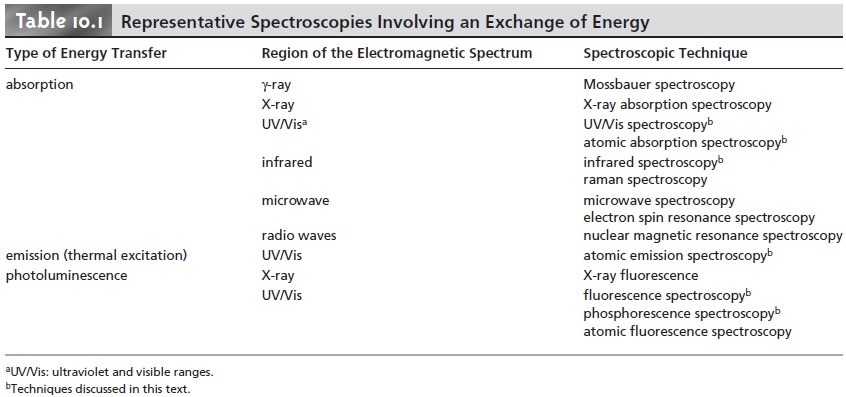
Spectroscopy is conveniently divided
into two broad
classes. In one
class of techniques, energy is transferred between a photon
of electromagnetic radiation and the analyte (Table 10.1). In absorption spectroscopy the energy carried
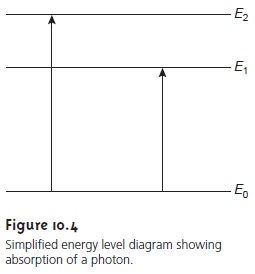
by a photon is absorbed by the analyte, promoting the analyte
from a lower-energy state to a higher-energy, or excited, state (Figure 10.4). The source of the energetic state de-
pends on the photon’s energy.
The electromagnetic spectrum
in Figure 10.3, for ex- ample, shows that absorbing a photon of visible light
causes a valence
electron in the analyte
to move to a higher-energy level. When an analyte absorbs
infrared radi- ation, on the other
hand, one of its chemical bonds experiences a change in vibra-
tional energy.
The intensity of photons passing
through a sample
containing the analyte
is at- tenuated because
of absorption. The
measurement of this
attenuation, which we call
absorbance, serves as our signal. Note that the energy levels
in Figure 10.4 have
well-defined values (i.e.,
they are quantized). Absorption only occurs
when the pho- ton’s energy matches the difference in energy, ∆E, between two energy levels.
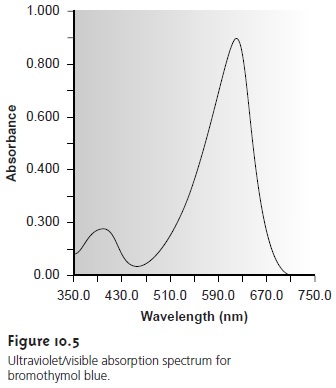
A plot of absorbance as a function
of the photon’s energy is called an absorbance
spec- trum (Figure 10.5).
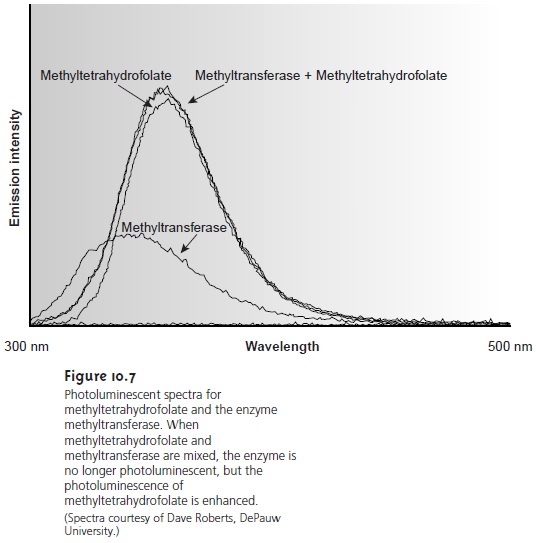
Emission of a photon occurs when an analyte in a higher-energy state returns to a lower-energy state (Figure 10.6). The higher-energy state can be achieved in several ways, including thermal energy, radiant energy from a photon, or by a chemical reaction.
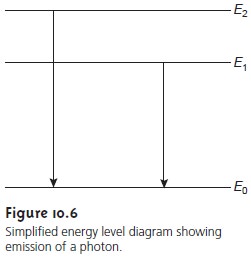
Emission following the absorption of a photon
is also called photoluminescence, and that following a chemical reaction
is called chemilumi-
nescence. A typical
emission spectrum is shown
in Figure 10.7.
In
the second broad
class of spectroscopy, the electromagnetic radiation under- goes a change in amplitude, phase angle, polarization, or direction of propagation
as a result of its
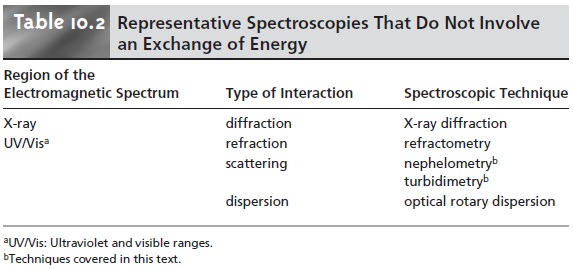
refraction, reflection, scattering, diffraction, or dispersion by the sample. Several
representative spectroscopic techniques are listed in Table 10.2.
Related Topics