Chapter: Modern Analytical Chemistry: Spectroscopic Methods of Analysis
Atomic Emission Spectra - Atomic Emission Spectroscopy
Atomic
Emission Spectra
Atomic emission occurs
when a valence electron in a higher-energy atomic orbital
returns to a lower-energy atomic
orbital. Figure 10.23
shows a portion
of the energy level diagram for sodium used earlier to discuss atomic
absorption spectra. An atomic emission spectrum, therefore, consists of a series of discrete lines at wave- lengths corresponding to the difference in energy between
two atomic orbitals.
The intensity, I, of
an emission line is proportional to the number of atoms, N*, populating the excited state
I =
kN* ………………
10.34
where k is a constant related
to the efficiency of the transition. For a system
in ther- mal equilibrium, the population of the excited
state is related
to the total
concentra- tion of atoms,
N, by the Boltzmann distribution. For many elements at tempera-
tures of less than 5000 K the Boltzmann distribution for the ith excited
state is approximated as
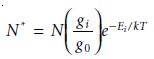 10.35
10.35
where
gi and g0 are statistical factors accounting for the number of equivalent energy levels for the excited state and ground state, Ei is the energy of the ex- cited state relative
to that of the ground state (E0 = 0), k is
Boltzmann’s constant
1.3807 x 10–23 J/K), and T is the temperature in kelvin. From equation
10.35 we can see that excited states with lower energies have larger populations and, therefore, the most intense emission lines. Furthermore, emission intensity in- creases
with temperature.
Related Topics