Chapter: Modern Analytical Chemistry: Spectroscopic Methods of Analysis
Equipment - Atomic Emission Spectroscopy
Equipment
Instrumentation for atomic
emission spectroscopy is similar in design to that used for
atomic absorption. In fact, most flame atomic
absorption spectrometers are eas-
ily adapted for use as flame atomic
emission spectrometers by turning off
the hol- low cathode
lamp and monitoring the difference between
the intensity of radiation
emitted when aspirating the sample
and that emitted
when aspirating a blank. Many atomic emission spectrometers,
however, are dedicated instruments designed to take advantage of features unique to atomic
emission, including the
use of plasmas, arcs, sparks, and lasers,
as atomization and excitation sources
and have an enhanced
capability for multielemental analysis.
Atomization and Excitation
Atomic emission requires
a means for converting an analyte in solid, liquid,
or solution form
to a free gaseous atom.
The same source
of thermal energy usually
serves as the excitation source.
The most common
methods are flames and
plasmas, both of which are
useful for liquid
or solution samples. Solid samples
may be analyzed by dissolving
in solution and using a flame or plasma
atomizer.
Flame Sources
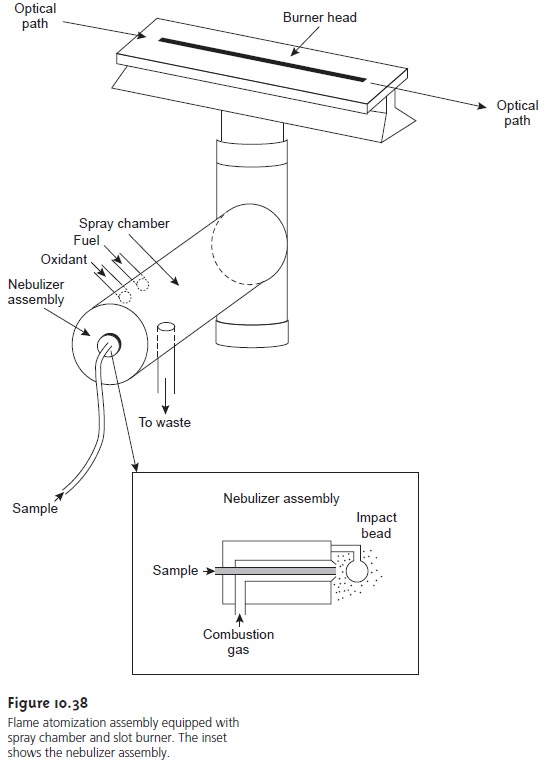
Atomization and excitation in flame atomic
emission is accom- plished using the same nebulization and spray chamber
assembly used in atomic absorption (see Figure
10.38). The burner
head consists of single or multiple slots or
a Meker-style burner.
Older atomic emission
instruments often used a total consumption burner
in which the
sample is drawn
through a capillary tube and in- jected directly into the flame.
Plasma Sources
A plasma consists of a hot,
partially ionized gas,
containing an abundant concentration of cations and electrons that make the plasma a conductor.
The plasmas used in atomic
emission are formed
by ionizing a flowing stream
of argon, producing argon
ions and electrons. The high temperatures in a plasma
re- sult from resistive
heating that develops
due to the movement of the electrons
and argon ions. Because
plasmas operate at much higher
temperatures than flames,
they provide better atomization and more highly populated excited
states. Besides neu- tral
atoms, the higher
temperatures of a plasma also produce ions of the analyte.
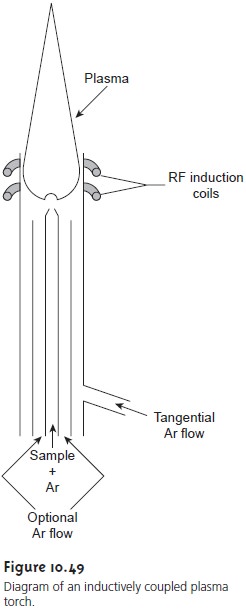
A schematic diagram of the inductively coupled plasma (ICP) torch is shown in Figure 10.49. The ICP torch consists of three concentric quartz tubes, sur- rounded at the top by a radio-frequency induction coil. The sample is mixed with a stream of Ar using a spray chamber nebulizer similar to that used for flame emission and is carried to the plasma through the torch’s central tube. Plasma formation is initiated by a spark from a Tesla coil. An alternating radio- frequency current in the induction coils creates a fluctuating magnetic field that induces the argon ions and electrons to move in a circular path.
The resulting collisions with the abundant unionized gas give rise to resistive heating, providing temperatures as high as 10,000 K at the base of the plasma,
and between 6000 and
8000 K at a height
of 15–20 mm above the coil, where
emission is usually measured. At these high temperatures the outer quartz
tube must be thermally
isolated from the plasma. This is accomplished by the tangential flow of argon shown in the schematic diagram.
Multielemental Analysis
Atomic emission
spectroscopy is ideally suited for multi- elemental analysis because all analytes in a sample
are excited simultaneously. A scanning monochromator can be programmed to move rapidly to
an analyte’s de- sired wavelength, pausing to record
its emission intensity before moving to the next analyte’s wavelength. Proceeding in this fashion,
it is possible to analyze
three or four analytes per minute.
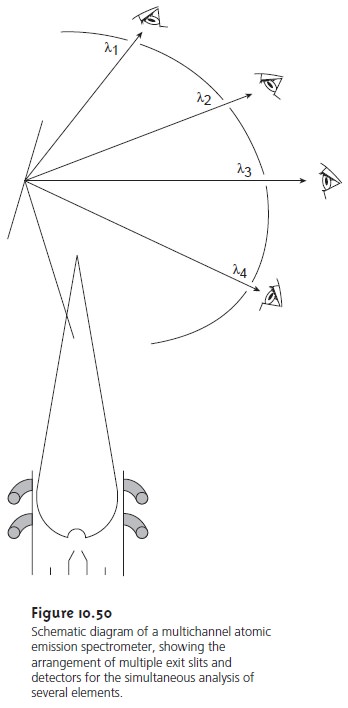
Another approach to multielemental analysis
is to use a multichannel instru- ment that allows
for the simultaneous monitoring of many
analytes. A simple
de- sign for a multichannel spectrometer consists of a standard diffraction grating and 48–60 separate exit slits and detectors positioned in a semicircular array around the diffraction grating
at positions corresponding to the desired
wave- lengths (Figure 10.50).
Related Topics