Chapter: 11th 12th std standard Class Physics sciense Higher secondary school College Notes
Capillarity and Illustrations of capillarity
Capillarity
The
property of surface tension gives rise to an interesting phenomenon called
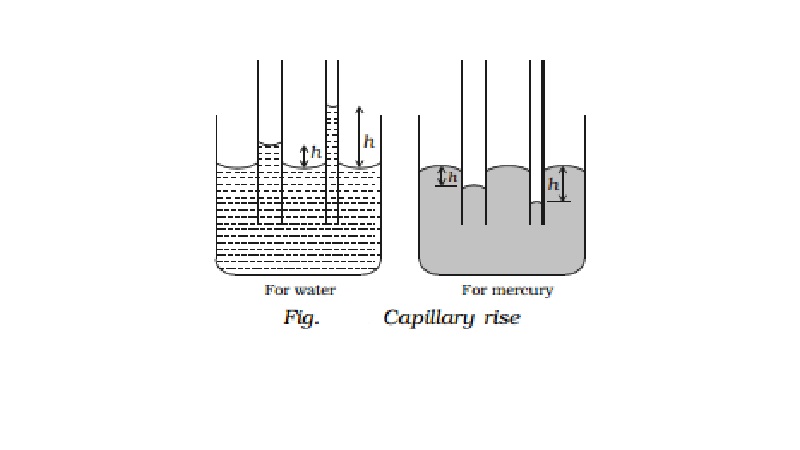
capillarity. When a capillary tube is dipped in water, the water rises up in
the tube. The level of water in the tube is above the free surface of water in
the beaker (capillary rise). When a capillary tube is dipped in mercury,
mercury also rises in the tube. But the level of mercury is depressed below the
free surface of mercury in the beaker (capillary fall).
The rise of a liquid in a capillary tube is known as
capillarity. The height h in Fig. indicates the capillary rise (for water) or
capillary fall (for mercury).
Illustrations of capillarity
(i) A blotting paper absorbs ink by capillary action. The pores in the
blotting paper act as capillaries.
(ii)
The oil in a lamp rises up the wick through the
narrow spaces between the threads of the wick.
(iii)
A sponge retains water due to capillary action.
(iv)
Walls get damped in rainy season due to
absorption of water by bricks.
Surface tension by capillary rise method
Let us
consider a capillary tube of uniform bore dipped vertically in a beaker
containing water. Due to surface tension, water rises to a height h in the
capillary tube as shown in Fig.. The surface tension T of the water acts
inwards and the reaction of the tube R outwards. R is equal to T in magnitude
but opposite in direction. This reaction R can be resolved into two rectangular
components.
(i)
Horizontal component R sin θ acting
radially outwards
(ii)
Vertical component R cos θ acting
upwards.
The
horizontal component acting all along the circumference of the tube cancel each
other whereas the vertical component balances the weight of water column in the
tube.
Related Topics