Chapter: 11th 12th std standard Class Physics sciense Higher secondary school College Notes
Millikan's oil drop experiment - Determination of charge of an electron
Determination of charge of an
electron - Millikan's oil drop experiment
Millikan's experiment is used for
the measurement of charge of an electron.
Principle
This method is based on the study of
the motion of uncharged oil drop under free fall due to gravity and charged oil
drop in a uniform electric field. By adjusting uniform electric field suitably,
a charged oil drop can be made to move up or down or even kept balanced in the
field of view for sufficiently long time and a series of observations can be
made.
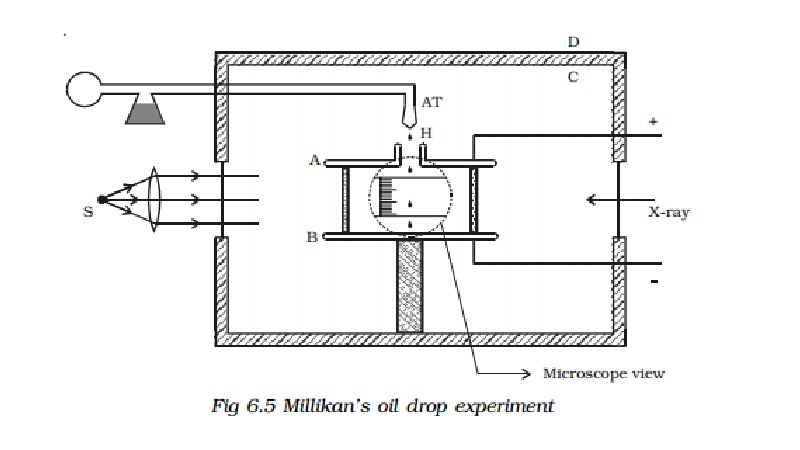
Experimental arrangement
The apparatus consists of two
horizontal circular metal plates A and B, about 22 cm in diameter and separated
by a distance of about 16 mm as shown in Fig 6.5. The upper plate has a hole
(H) in the middle. These plates are held together by insulating rods of glass
or ebonite, so that they are perfectly parallel to each other.
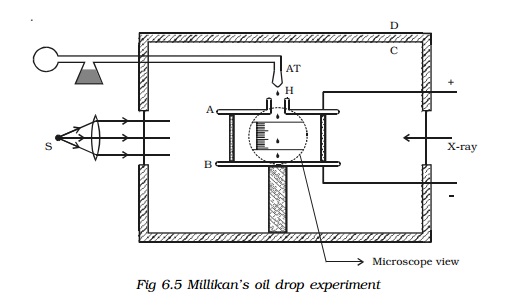
The plates are surrounded by a
constant temperature bath D and the chamber C containing dry air. The plates
are connected to a battery which can provide a potential difference of the
order of 10000 V.
Theory
A spray of fine droplets of a highly
viscous liquid (such as glycerine) is produced by means of an atomiser (AT)
near the hole H and enter the space between A and B. The droplets are
illuminated by an arc lamp L and are seen through a microscope whose eyepiece
is provided with a micrometer scale. One such droplet is viewed through the
microscope as it descends under gravity. The viscous force due to air increases
and soon it attains a constant terminal velocity and let it be v. The terminal velocity v of the droplet is measured using the
microscope.
(i)
Motion under gravity
The gravitational force acting on
the oil drop downwards is equal to mg
=4/3 πa3 ρ g, where a is the
radius of the oil drop, ρ is the density of the oil and g, the acceleration due to gravity.
The
upthrust experienced by the oil drop due to the displaced air is 4/3 πa3
σ g, where σ is the density of air.
∴ The net downward force
acting on the oil drop = weight of the oil drop - upthrust experienced by the
oil drop.
=(
4/3 πa3 ρ g ) - ( 4/3 πa3 σ g )
=
4/3 πa3 (ρ - σ) g ……………….(1)
Since
the oil drop attains a terminal velocity v, the net downward force acting on
the oil drop is equal to the viscous force acting opposite to the direction of
motion of the oil drop.
By
Stoke's law, the viscous force on the oil drop is 6πaηv, where η is the
co-efficient of viscosity of air.
4
/ 3 πa3 (ρ
- σ) g = 6πaηv ………….(2)
The
radius of the oil drop is,
A=
[9ηv / 2(ρ - σ)g ]1/2 ………… (3)
(ii) Motion under electric field
The air inside the parallel plates
is ionized by sending a beam of X-rays. The droplets pickup one or more
electrons from the ionized air.
Let q be the charge carried by the droplet under observation. Let E be the electric field applied between
the plates A and B, so that the drop
moves upwards with a terminal velocity v1,
which can be determined using the microscope.
The force on the droplet due to
electric field is Eq. Since the
velocity of the droplet is uniform, we have
Eq =
4/3 πa3
( ρ - σ ) g + 6π aηv1
Eq
- 4/3 πa3
( ρ - σ ) g
= 6π aηv1 ……………….. (4)
Adding equations (2) and (4),
Eq
=
6π aη(v + v1)
……………. (5)
Substituting the value of a in equation (5) from equation (3),
Eq
=
6πη3/2(v
+ v1 )[9v/2( ρ - σ ) g]1/2
If V is the potential difference
between A and B, d is the distance between them, then E =V/d
Millikan determined the value q for a large number of oil drops using
equation (6) and found that they are an integral multiple of a least value. The
greatest common factor gives the charge e of the electron.
The charge of an electron was found
to be 1.602 × 10-19 coulomb.
Related Topics