Chapter: 11th 12th std standard Class Physics sciense Higher secondary school College Notes
Rutherford atom model and its Drawbacks
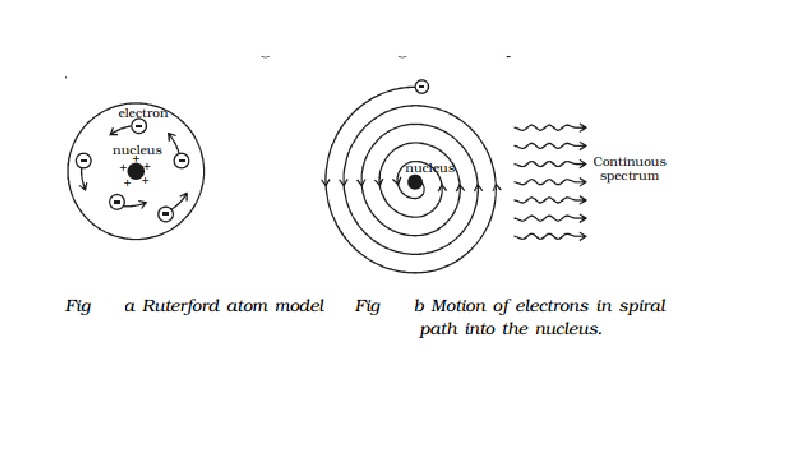
Rutherford atom model
Based on the results of α-particle scattering experiment,
Rutherford suggested the following picture of the atom.
1)
Atom may be regarded as a sphere of
diameter 10-10m, but whole of the positive charge and almost the
entire mass of the atom is concentrated in a small central core called nucleus
having diameter of about 10-14m as shown in Fig a.
2)
The electrons in the atom were
considered to be distributed around the nucleus in the empty space of the atom.
If the electrons were at rest, they would be attracted and neutralized by the
nucleus. To overcome this, Rutherford suggested that the electrons are
revolving around the nucleus in circular orbits, so that the centripetal force
is provided by the electrostatic force of attraction between the electron and
the nucleus.
3)
As the atom is electrically neutral,
the total positive charge of the nucleus is equal to the total negative charge
of the electrons in it.
Drawbacks
Rutherford atom model offered serious difficulties as
regards the stability of the atom. Following are the two drawbacks of
Rutherford's model: (i) The electron in the circular orbit experiences a
centripetal acceleration. According to electromagnetic theory, an accelerated electric
charge must radiate energy in the form of electromagnetic waves. Therefore, if
the accelerated electron lose energy by radiation, the energy of the electron
continuously decreases and it must spiral down into the nucleus, as shown in
Fig 6.9b. Thus, the atom cannot be stable. But, it is well known that most of
the atoms are stable. (ii) According to classical electromagnetic theory, the
accelerating electron must radiate energy at a frequency proportional to the
angular velocity of the electron. Therefore, as the electron spiral towards the
nucleus, the angular velocity tends to become infinity and hence the frequency
of the emitted energy will tend to infinity. This will result in a continuous
spectrum with all possible wavelengths. But experiments reveal only line
spectra of fixed wavelength from atoms.
Related Topics