Chapter: 11th 12th std standard Class Physics sciense Higher secondary school College Notes
Bragg's X-ray spectrometer
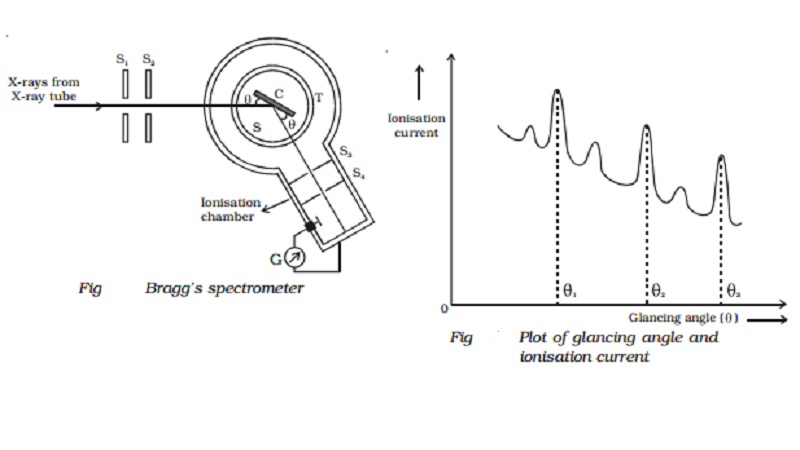
Bragg's X-ray spectrometer
Bragg's spectrometer used to determine the
wavelength of X - rays is shown in Fig. Bragg's spectrometer is similar in
construction to an ordinary optical spectrometer.
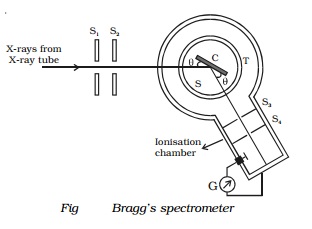
X-rays from an X-ray tube are made to pass
through two fine slits S1 and S2 which collimate it into
a fine pencil. This fine X-ray beam is then made to fall upon the
crystal 'C' (usually sodium chlo-
ride crystal) mounted on the
spectrometer table. This table is capable of rotation
about a vertical axis and its rotation can be read on a circular
graduated scale S. The reflected beam
after passing through the slits S3
and S4 enters the
ionization chamber. The X-rays entering the ionization chamber ionize the gas
which causes a current to flow between the electrodes and the current can be
measured by galvanometer G. The ionization current is a measure of the
intensity of X-rays reflected by the crystal.
The ionization current is measured for different
values of glancing angle θ. A
graph is drawn between the glancing angle θ and ionization current (Fig.).
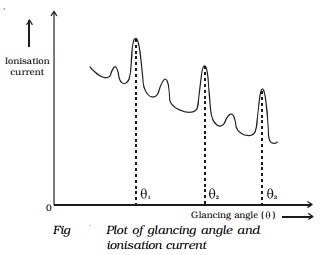
For
certain values of glancing angle,the ionization
current increases abruptly. The first peak corresponds to first order, the second peak to second
order and so on. From the graph, the glancing angles for different orders of
reflection can be measured. Knowing the angle θ and the spacing d for the crystal, wavelength of X-rays can be determined.
Related Topics