Chapter: 11th 12th std standard Class Physics sciense Higher secondary school College Notes
X-ray spectra - continuous and characteristic X-ray spectra
X-ray spectra - continuous and characteristic X-ray
spectra.
The spectrum from an X-ray tube contains two
distinct parts :
(i) Continuous X-ray spectra
It consists of radiations of all possible wavelengths,
from a certain lower limit to higher values continuously, as in the case of
visible light.
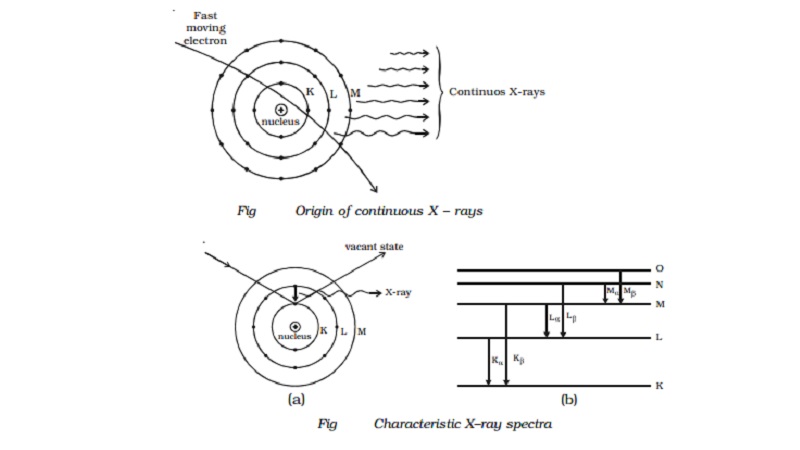
Origin - Continuous X-ray spectra
X-rays are produced, when high velocity
electrons strike the target material of high atomic number. It has also been
mentioned in the production of X-rays, that most of the energy of the electrons
goes into the heating of the target material.
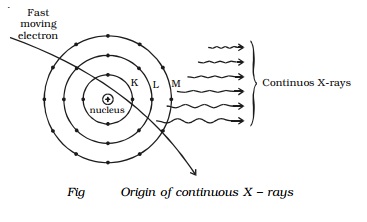
A few fast moving electrons penetrate deep into
the interior of the atoms of the target material and are attracted towards the
nuclei by the attractive forces of their nuclei. Due to these forces, the
electrons get deflected from their original path. As a result of this, the
electrons are decelerated, and hence energy of the electron decreases
continuously. This loss of energy during retardation is given off in the form
of X-rays of continuously varying wavelength. The X - rays consist of
continuous range of frequencies upto a maximum frequency ímax or minimum wave length λmin. This
is called as continuous X - rays. The minimum wave length depends on the anode
voltage. If V is the potential
difference between the anode and the cathode
eV = hνmax = hc / λmin
The
minimum wavelength of the given radiation is,
λmin
= hc /eV
where
h is Planck's constant, c is the velocity of light and e, the charge of the
electron. Substituting the known values in the above equation.
λmin
= 12400/V A0
For the given operating voltage, the minimum
wave length is same for all metals.
(ii) Characteristic X-ray spectra
It consists of definite, well defined
wavelengths superimposed on the continuous spectrum. These spectral lines
generally occur in the form of small groups and are characteristic of the
material of the target.

Origin - Characteristic X-ray spectra
Few of the fast moving electrons having
velocity of about (1/10)th of the velocity of light may penetrate
the surface atoms of the target materials and knock out the tightly bound
electrons even from the inner most shells (like K, L shells) of the atom. Fig
6.22a shows the case, when the fast moving electrons knock off one electron
from K-Shell and the vacancy is filled by the nearby electron from the L shell.
During this transition, the energy difference is radiated in the form of X-rays
of very small wave length. This corresponds to Kα - line of the series. The frequency ν1 of this line is given by the relation (EK - EL)
= hν1.
Suppose, the electron from M shell jumps to the K shell, it gives out Kβ line and so on. If an electron jumps from the M-Shell to the
vacant state in L-Shell, it contributes Lα line and if the vacancy in L-Shell is filled
up by an electron of N shell, it contributes Lβ and so on (Fig 6.22b). The frequency of radiation depends upon the
target material. The X-ray spectra consists of sharp lines and is the
characteristic of target material. Hence this spectra is known as
characteristic spectra.
Related Topics