Chapter: 11th 12th std standard Class Physics sciense Higher secondary school College Notes
Photoelectric effect - Dual Nature of Radiation
Dual Nature of Radiation and Matter and Relativity
The true nature of light is
difficult to assess. Experiments showed that light exhibited wavelike
properties of diffraction and interference. On the other hand, photoelectric
effect indicates that light has the aspects of a particle photon, with both
energy and momentum. Thus light exhibits a wave-particle duality.
The wave-particle duality was
extended to particles as matter waves by Louis de Broglie. His theoretical
study on the nature of particles and waves led to the invention of a new
mechanics of particles called quantum mechanics.
Photoelectric effect
Photoelectric emission is the
phenomena by which a good number of substances, chiefly metals, emit electrons
under the influence of radiation such as γ rays, X-rays, ultraviolet and even visible
light. This effect was discovered by Heinrich Hertz in 1887 while working with
resonance electrical circuits. A year later, Hallwachs, Elster and Geitel
investigated the phenomenon with a simple experimental arrangement.
Hallwachs
Experiment
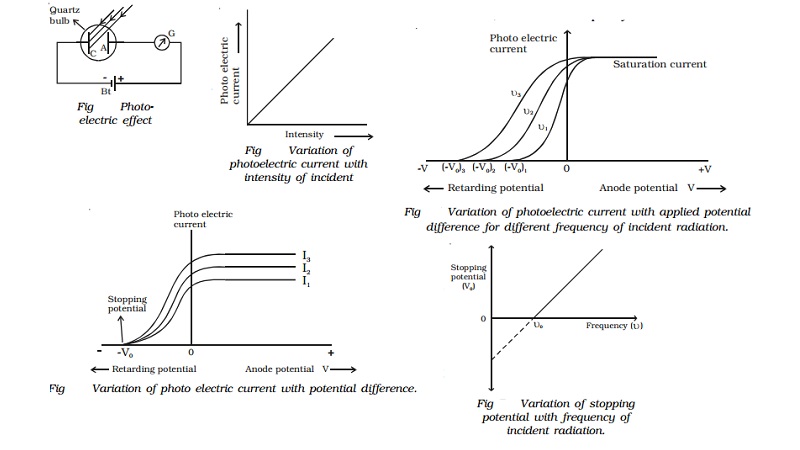
Hallwachs experimental set-up to study the
photo electric effect is shown in Fig It
consists of an evacuated quartz bulb with two zinc plates cathode C and anode
A. The plates are connected to a battery and a sensitive galvanometer. In the
absence of any
radiation incident on the
plates, there is no flow of current and hence there is no deflection in the
galvanometer.
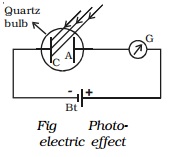
But, when an electro magnetic radiation like
ultraviolet radiation is allowed to fall on the plate C which is connected to
the negative terminal of the battery, a current begins to flow, indicated by
the deflection in the galvanometer (G). But, when ultraviolet radiation is made
to fall on A, there is no deflection in the galvanometer. These observations
reveal that the particles emitted by the plate C due to the photoelectric
effect are negatively charged. These particles were found to be electrons. The
observed current known as the photoelectric current is due to the flow of
electrons.
After the study of photoelectric effect by
Hallwachs, scientists J.J.Thomson, Lenard, Richardson, Compton did a series of
experiments to study the relationship between photoelectric current, intensity
of incident radiation, velocity and the kinetic energy of the photo electrons,
and their dependence on the wave length of incident radiation used.
Effect of intensity of incident radiation on
photo electric current
Keeping the frequency of the Photo electric incident radiation and the potential difference between the cathode and the anode at constant values, the intensity of incident radiation is varied. The corresponding photoelectric current is measured in the microammeter.
It
is found that the photo electric current increases
linearly with the intensity of incident radiation (Fig).
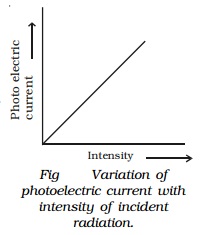
Since the photoelectric current is directly
proportional to the number of photoelectrons emitted per second, it implies
that the number of photoelectrons emitted per second is proportional to the
intensity of incident radiation.
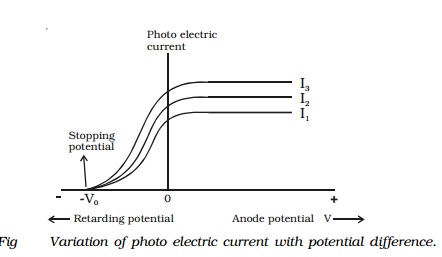
Effect of potential difference on the
photoelectric current
For a given metallic surface C, keeping the
intensity (I1) and
frequency of the incident radiation constant, the effect of potential
difference between the plates on the photoelectric current can be studied. Fig
7.3 shows the variation of photo electric current with the potential difference
V between the two plates. When the positive potential of A is increased, the
photoelectric current is also increased. However, if the positive potential is
further increased such that it is large enough to collect all the photo
electrons emitted from the plate C, the photoelectric current reaches a certain
maximum value and this current is known as saturation current. If the potential
of the plate A is made negative, the photocurrent does not immediately drop to
zero but flows in the same direction as for positive potential. This shows that
the photo electrons are emitted from the plate C with finite velocity. If the
negative or retarding potential is further increased, the photo current
decreases and finally becomes zero at a particular value. Thus, the minimum
negative (retarding) potential given to the anode for which the photo electric
current becomes zero is called the cut-off or stopping potential.
If m
is the mass of the photo electron emitted with a velocity vmax then the kinetic energy associated with it is ½ mv2max.
Since at the stopping potential Vo,
the fastest electron is just prevented from reaching the plate A, workdone in
bringing the fastest electron to rest = kinetic energy of the fastest electron.
Since at the stopping potential Vo, the fastest electron is just
prevented from reaching the plate A, workdone in bringing the fastest electron
to rest = kinetic energy of the fastest electron.
eVo
= ½ mv2max
The above equation indicates that the stopping
potential depends upon the velocity of the fastest electron.
The experiment is repeated with the incident
radiation of same frequency, but of higher intensities I2 and I3.
It is found from the graph, the saturation currents are proportional to the
intensities of the radiation. But, the stopping potential remains the same for
all the intensities. Thus, for a given frequency of incident radiation, the
stopping potential is independent of its intensity.
Effect of frequency of incident radiation on
stopping potential
Keeping the photosensitive plate (C) and
intensity of incident radiation a constant, the effect of frequency of the
incident radiations on stopping potential is studied. Fig 7.4 shows the
variation of the photo electric current with the applied potential difference V for three different frequencies. From
the graph, it is found that higher the frequency of the incident radiation,
higher is the value of stopping potential Vo.
For frequencies ν3 > ν2 > ν 1, the corresponding stopping
potentials are in the same order (Vo
)3 > (Vo )2
> (Vo )1.
It is concluded from the graph that, the
maximum kinetic energy of the photoelectrons varies linearly with the frequency
of incident radiation but is independent of its intensity.
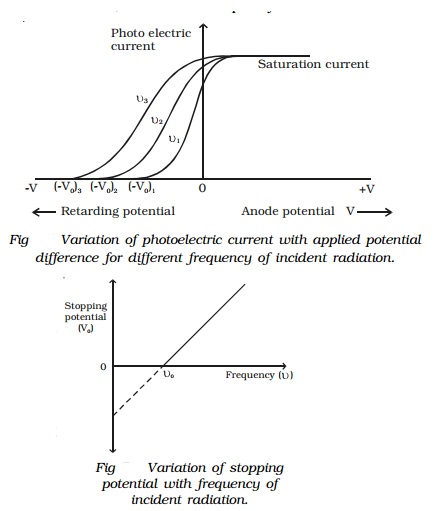
IIf the frequency of the incident radiation is
plotted against the corresponding stopping potential, a straight line is
obtained as shown in Fig 7.5. From this graph, it is found that at a frequency νo, the
value of the stopping potential is zero. This frequency is known as the
threshold frequency for the photo metal used. The photoelectric effect occurs
above this frequency and ceases below it. Therefore, threshold frequency is
defined as the minimum frequency of incident radiation below which the
photoelectric emission is not possible completely, however high the intensity
of incident radiation may be. The threshold frequency is different for
different metals.
Laws of photoelectric emission
The experimental observations on photoelectric
effect may be summarized as follows, which are known as the fundamental laws of
photoelectric emission.
1. For a given photo sensitive material, there is
a minimum frequency called the threshold frequency, below which emission of
photoelectrons stops completely, however great the intensity may be.
2. For a given photosensitive material, the photo
electric current is directly proportional to the intensity of the incident
radiation, provided the frequency is greater than the threshold frequency.
3. The photoelectric emission is an instantaneous
process. i.e. there is no time lag between the incidence of radiation and the
emission of photo electrons.
4. The maximum kinetic energy of the photo
electrons is directly proportional to the frequency of incident radiation, but
is independent of its intensity.
Light waves and photons
The electromagnetic theory of light proposed by
Maxwell could not explain photoelectric effect. But, Max Planck's quantum
theory successfully explains photoelectric effect. According to Planck's
quantum theory, light is emitted in the form of discrete packets of energy
called 'quanta' or photon. The energy of each photon is E = hν, where h
is Planck's constant.
Photon is neither a particle nor a wave. In the
phenomena like interference, diffraction, polarisation, the photon behaves like
a wave. In the phenomena like emission, absorption and interaction with matter
(photo electric effect) photon behaves as a particle. Hence light photon has a
dual nature.
Einstein's photoelectric equation
In 1905, Albert Einstein, successfully applied
quantum theory of radiation to photoelectric effect.
According to Einstein, the emission of photo
electron is the result of the interaction between a single photon of the
incident radiation and an electron in the metal. When a photon of energy hν is incident on a metal surface, its energy is used up in two ways
:
1. A part of the energy of the photon is used in
extracting the electron from the surface of metal, since the electrons in the
metal are bound to the nucleus. This energy W spent in releasing the photo
electron is known as photoelectric work function of the metal. The work
function of a photo metal is defined as the minimum amount of energy required
to liberate an electron from the metal surface.
2. The remaining energy of the photon is used to
impart kinetic energy to the liberated electron.
If m
is the mass of an electron and v, its
velocity then
Energy of the incident photon = Work function +
Kinetic energy of the electron
hν = W + ½ mv2 …… (1)
If the electron does not lose energy by
internal collisions, as it escapes from the metal, the entire energy (hν-W) will be exhibited as the kinetic energy of the electron. Thus, (hν-W) represents the maximum kinetic energy of the ejected photo
electron. If vmax is the
maximum velocity with which the photoelectron can be ejected, then
hν = W + ½ mvmax2 …… (1)
This equation is known as Einstein's
photoelectric equation.
When the frequency (ν) of the incident radiation is equal to the threshold frequency (νo) of the
metal surface, kinetic energy of the electron is zero. Then equation (2)
becomes,
hνo = W …………………..(3)
Substituting the value of W in equation (2) we get,
hν - hνo = ½ mv2max
or
h(v-v0)= ½ mv2max
This is another form of Einstein's photoelectric
equation.
Experimental verification of Einstein's
photoelectric equation
Einstein's photoelectric equation is,
½ mv2max = h(v-v0)
½ mv2max = eV0
From equations (1) and (2)
eV0 = h(v-v0)
V0= (h/e)v - (h/e)v0 ………………………(3)
This is an equation of a straight line.
Millikan verified equation (3) experimentally and found that it is in harmony
with the observed facts.
Related Topics