Chapter: Modern Analytical Chemistry: Equilibrium Chemistry
Thermodynamics and Equilibrium Chemistry
Thermodynamics and
Equilibrium Chemistry
Thermodynamics is the
study of thermal,
electrical, chemical, and
mechanical forms of energy. The study of thermodynamics crosses
many disciplines, including physics, engineering, and chemistry. Of the various
branches of thermodynamics the most important to chemistry is the study
of the changes
in energy occurring during a chemical reaction.
Consider, for example,
the general equilibrium reaction shown in equation 6.1, involving the solutes A, B, C, and D, with stoichiometric coefficients a, b, c, and d.
aA+ bB
< = = = = > cC+ dD
By convention, species
to the left of the arrows are called reactants, and those on the right
side of the arrows are called products. As Berthollet discovered, writing a reac- tion in this fashion
does not guarantee that the reaction
of A and B to produce C and
D is
favorable. Depending on initial conditions, the reaction may move to the left, to
the right, or be in a state
of equilibrium. Understanding the factors that determine
the final position of a reaction is one of the goals
of chemical thermodynamics.
Chemical systems spontaneously react in a fashion that
lowers their overall
free energy. At a constant temperature and pressure, typical
of many bench-top chemi- cal reactions, the free energy
of a chemical reaction is given by the GibbŌĆÖs free en-
ergy function
ŌłåG = ŌłåH ŌĆō T ŌłåS
where T is the temperature in kelvins, and ŌłåG, ŌłåH, and ŌłåS are
the differences in the GibbŌĆÖs
free energy, the enthalpy, and the entropy
between the products
and reactants.
Enthalpy is
a measure of the net
flow of energy,
as heat, during
a chemical re- action. Reactions in which heat is produced have a negative
ŌłåH and are called exothermic. Endothermic reactions absorb heat
from their surroundings and have a positive ŌłåH. Entropy is
a measure of randomness, or disorder. The entropy of an
individual species is always positive
and tends to be larger
for gases than for solids and for more complex
rather than simpler
molecules. Reactions that result in a
large number of simple, gaseous
products usually have a positive
ŌłåS.
The sign of ŌłåG can be used to predict the
direction in which
a reaction moves to reach its equilibrium position.
A reaction is always thermodynamically favored when enthalpy decreases and entropy increases. Substituting the inequalities ŌłåH <0
and ŌłåS > 0 into equation
6.2 shows that ŌłåG is negative
when a reaction is thermo- dynamically favored. When ŌłåG is positive, the reaction is unfavorable as written
(although the reverse
reaction is favorable). Systems at equilibrium have a ŌłåG of zero.
As a system
moves from a nonequilibrium to an equilibrium position, ŌłåG must
change from its initial value
to zero. At the same
time, the species
involved in the reaction undergo a change in their concentrations.
The GibbŌĆÖs free energy, there- fore, must be a function
of the concentrations of reactants and products.
As shown in equation 6.3, the GibbŌĆÖs
free energy can be divided
into two terms.
ŌłåG =
ŌłåG┬░+ RT ln
Q ŌĆ”ŌĆ”ŌĆ”ŌĆ”6.3
The first term,
ŌłåG┬░, is the change
in GibbŌĆÖs free
energy under standard-state condi- tions; defined as a temperature of 298 K, all gases
with partial pressures of 1 atm, all
solids and liquids pure, and all solutes
present with 1 M concentrations. The second term, which
includes the reaction quotient, Q, accounts for
nonstandard-state pres-
sures or concentrations. For reaction 6.1 the reaction quotient is
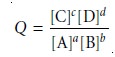
where the terms in brackets
are the molar concentrations of the solutes.
Note that the reaction
quotient is defined
such that the concentrations of products are placed
in the numerator, and the concentrations of reactants are placed in the denominator. In addition, each concentration term is raised to a power equal to its stoichiometric
coefficient in the balanced chemical
reaction. Partial pressures
are substituted for concentrations when the reactant or
product is a gas. The concentrations of pure
solids and pure liquids do not change during a chemical reaction
and are excluded from the reaction
quotient.
At equilibrium the GibbŌĆÖs free energy is zero, and equation 6.3
simplifies to
ŌłåG┬░=
ŌĆōRT ln K
where K is an equilibrium constant that defines
the reactionŌĆÖs equilibrium posi- tion. The equilibrium constant is just
the numerical value
obtained when substitut- ing the concentrations of reactants and products at equilibrium into equation 6.4; thus,
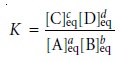
where the subscript ŌĆ£eqŌĆØ indicates a concentration at equilibrium. Although
the subscript ŌĆ£eqŌĆØ is usually omitted, it is important to remember that
the value of K is
determined by the concentrations of solutes at equilibrium.
As written, equation 6.5 is a limiting law
that applies only
to infinitely dilute solutions, in which the chemical behavior
of any species in the system is unaffected
by all other species.
Related Topics