Chapter: Modern Analytical Chemistry: Equilibrium Chemistry
Equilibrium Constants for Acid–Base Reactions
Acid–Base Reactions
A useful definition
of acids and bases is that independently introduced by Jo- hannes
Brønsted (1879–1947) and Thomas Lowry
(1874–1936) in 1923.
In the Brønsted-Lowry definition, acids are
proton donors, and
bases are proton accep- tors. Note that these
definitions are interrelated. Defining a base as a proton accep- tor means an acid must be available to provide the proton. For example, in reac-
tion 6.7 acetic acid, CH3COOH, donates
a proton to ammonia, NH3, which serves as the base.
CH3COOH(aq)+
NH3(aq) < = = = = > CH3COO–(aq)+ NH4+(aq) 6.7
|
4 |
Strong and Weak Acids
The reaction of an acid with its solvent (typically water) is called an acid dissociation reaction. Acids are divided into two categories based on the ease
with which they
can donate protons
to the solvent. Strong acids, such
as HCl, almost completely transfer their protons
to the solvent molecules.
HCl(aq)+ H2O(l) → H3O+(aq)+ Cl–(aq)
In this reaction H2O serves as the base.
The hydronium ion,
H3O+, is the
conju- gate acid of H2O, and
the chloride ion
is the conjugate base of HCl.
It is the hy- dronium ion that is the acidic
species in solution, and its concentration deter- mines the
acidity of the
resulting solution. We have chosen
to use a single arrow (→)
in place of the double arrows (t) to indicate that we treat HCl as if it were completely dissociated in aqueous solutions. A solution of 0.10 M HCl is effec-
tively 0.10 M in H3O+ and
0.10 M in Cl–. In aqueous
solutions, the common strong acids are hydrochloric acid (HCl), hydroiodic acid (HI), hydrobromic acid (HBr), nitric acid (HNO3), perchloric acid (HClO4), and the first proton of sulfu-
ric acid (H2SO4).
Weak acids, of which aqueous
acetic acid is one example, cannot completely
donate their acidic protons to the solvent.
Instead, most of the acid remains undis- sociated, with only a small fraction
present as the conjugate base.
CH3COOH(aq)+ H2O(l) < = = = = > H3O+(aq)+ CH3COO–(aq)
The equilibrium constant for this
reaction is called an acid dissociation
constant,
Ka, and is written as
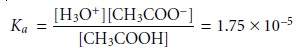
Note that the concentration of H2O is omitted from the Ka expression because its value
is so large that it is unaffected by the dissociation reaction.* The magnitude of Ka
provides information about the relative strength of a weak acid, with a
smaller Ka corresponding to a weaker acid. The ammonium
ion, for example, with a Ka of 5.70 x 10–10, is a weaker
acid than acetic
acid.
Monoprotic weak acids,
such as acetic
acid, have only a single
acidic proton and a single acid dissociation constant.
Some acids, such as phosphoric acid, can donate more than one proton and are called
polyprotic weak acids.
Polyprotic acids are described by a series
of acid dissociation steps, each characterized by it own
acid dissociation constant. Phosphoric acid, for example,
has three acid dissociation re- actions and acid dissociation constants.

The decrease in the acid dissociation constant
from Ka1 to Ka3 tells
us that each suc-
cessive proton is harder to remove. Consequently, H3PO4
is a stronger acid than H2PO4–,
and H PO4– is a stronger acid
than HPO42–.
Strong and Weak Bases
Just as the acidity of an aqueous
solution is a measure of the
concentration of the hydronium ion, H3O+, the basicity of an aqueous
solution is a measure
of the concentration of the hydroxide ion, OH–. The most common example of a strong
base is an alkali metal
hydroxide, such as sodium hydroxide, which completely dissociates to produce the hydroxide ion.
NaOH(aq) → Na+(aq)+ OH–(aq)
Weak bases only partially accept protons from the solvent
and are characterized by a base dissociation constant, Kb. For example, the
base dissociation reaction and base dissociation constant
for the acetate ion are

Polyprotic bases, like polyprotic acids,
also have more than one base dissociation re- action and base dissociation constant.
|
3 |
Amphiprotic Species
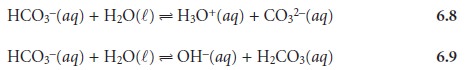
A species that
can serve as both a proton donor
and a proton acceptor is called am-
phiprotic. Whether an amphiprotic species
behaves as an acid or as a base depends on the equilibrium constants for the two competing reactions. For bicarbonate, the acid
dissociation constant for reaction 6.8
Ka2 = 4.69 x 10–11
is smaller than the base dissociation constant for reaction 6.9.
Kb2 = 2.25 x 10–8
|
3 |
Dissociation of Water
Water is an amphiprotic solvent
in that it can serve
as an acid or a base. An interesting feature of an amphiprotic solvent
is that it is capable of reacting with itself as an acid and a base.
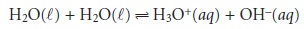
The equilibrium constant
for this reaction
is called water’s
dissociation con- stant, Kw,

which has a value of 1.0000 x 10–14 at a temperature of 24 °C. The value
of Kw varies
substantially with temperature. For example, at 20 °C, Kw is 6.809 x 10–15, but at
30
°C Kw is 1.469 x 10–14. At the standard
state temperature of 25 °C, Kw is 1.008 x 10–14,
which is sufficiently close to 1.00
x 10–14
that the latter
value can be used with negligible error.
The pH Scale
An important consequence of equation
6.10 is that the concentra- tions of H3O+ and OH– are related.
If we know [H3O+] for a solution, then [OH–] can be calculated using
equation 6.10.
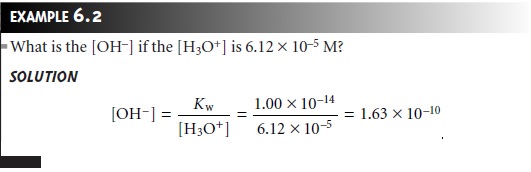
Equation 6.10 also allows us to develop
a pH scale that indicates the acidity of a so- lution. When the concentrations of H3O+ and OH– are equal,
a solution is neither
acidic nor basic; that is, the solution
is neutral. Letting
[H3O+] = [OH–]
and substituting into equation 6.10 leaves us with
Kw = [H3O+]2 = 1.00 x 10–14
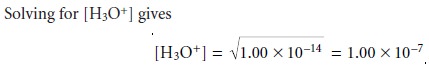
A neutral solution has a hydronium ion concentration of 1.00 x 10–7 M
and a pH of 7.00.* For
a solution to be acidic,
the concentration of H3O+ must be greater than that for OH–, or
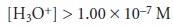
The pH of an acidic
solution, therefore, must be less than 7.00.
A basic solution, on the other hand,
will have a pH greater
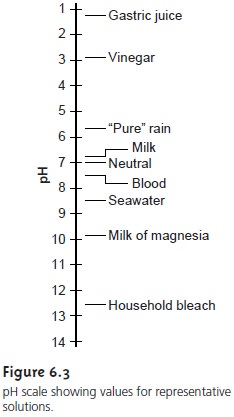
than 7.00. Figure
6.3 shows the
pH scale along with pH values
for some representative solutions.
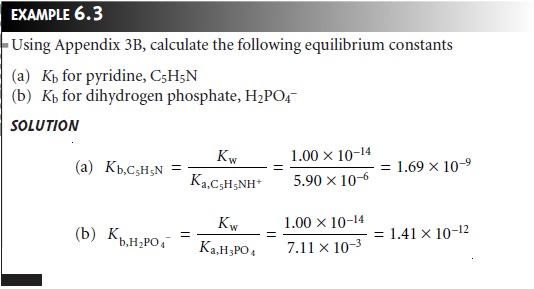
Tabulating Values for Ka and Kb
A useful
observation about acids
and bases is that
the strength of a base is inversely proportional to the strength of its conjugate acid. Consider, for example,
the dissociation reactions of acetic acid and acetate.
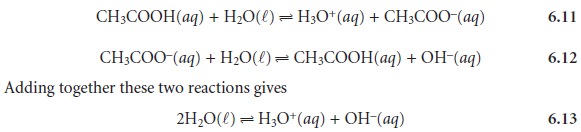
The equilibrium constant
for equation 6.13 is Kw. Since equation 6.13 is obtained by adding together reactions 6.11 and 6.12,
Kw may also be expressed as the product of Ka for
CH3COOH and Kb for
CH3COO–. Thus, for
a weak acid,
HA, and its
con- jugate weak base, A–,

This relationship between
Ka and Kb simplifies the tabulation of acid and base dis- sociation constants. Acid dissociation constants for a variety of weak acids
are listed in Appendix
3B. The corresponding values of Kb for their conjugate weak bases are determined using equation 6.14.
Related Topics