Chapter: Modern Analytical Chemistry: Equilibrium Chemistry
A Simple Problem: Solubility of Pb(IO3)2 in Water
A Simple Problem: Solubility of Pb(IO3)2
in Water
When an insoluble compound
such as Pb(IO3)2 is added to a solution
a small por- tion of the solid
dissolves. Equilibrium is achieved when the concentrations of Pb2+ and IO3– are sufficient to satisfy the solubility product
for Pb(IO ) . At equilibrium
the solution is saturated with Pb(IO3)2. How can we determine the concentrations
of Pb2+ and IO3–, and the solubility of Pb(IO ) in a saturated solution
prepared by adding Pb(IO3)2 to distilled water?

As equilibrium is established, two IO3– ions are produced for each ion of Pb2+.
If we assume that the molar
concentration of Pb2+ at equilibrium is x then the molar con- centration of IO3– is 2x. To
help keep track
of these relationships, we can use
the following table.
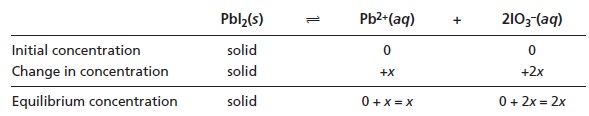
Substituting the equilibrium concentrations into equation 6.33

The equilibrium concentrations of Pb2+ and IO3–, therefore,
are
[Pb2+]= x = 4.0 x 10–5 M
[I–]= 2x = 7.9 x 10–5 M
Since one mole
of Pb(IO3)2 contains
one mole of Pb2+, the
solubility of Pb(IO3)2 is the same as the concentration of Pb2+; thus, the solubility of Pb(IO3)2 is 4.0
x 10–5 M.
Related Topics