Chapter: Modern Analytical Chemistry: Equilibrium Chemistry
Reversible Reactions and Chemical Equilibria
Reversible Reactions and Chemical Equilibria
In 1798, the chemist Claude
Berthollet (1748–1822) accompanied a French military expedition to Egypt. While
visiting the Natron
Lakes, a series
of salt water
lakes carved from limestone, Berthollet made an observation that contributed to an im- portant discovery. Upon analyzing
water from the Natron Lakes, Berthollet found
large quantities of common salt,
NaCl, and soda
ash, Na2CO3, a result he found sur- prising. Why would Berthollet find this result surprising and how did it contribute
Berthollet “knew” that
a reaction between
Na2CO3 and CaCl2 goes to comple- tion, forming NaCl and
a precipitate of CaCO3 as products.
Na2CO3 + CaCl2 → 2NaCl + CaCO3
Understanding this, Berthollet expected that large quantities of NaCl and Na2CO3
could not coexist in the presence of CaCO3. Since
the reaction goes to completion, adding a large quantity
of CaCl2 to a solution
of Na2CO3 should
produce NaCl and CaCO3, leaving
behind no unreacted Na2CO3.
In fact, this result is what he ob-
served in the laboratory. The evidence from Natron Lakes,
where the coexistence of NaCl and Na2CO3 suggests that the reaction has not gone to completion, ran counter to Berthollet’s expectations. Berthollet’s important insight
was recognizing that the chemistry occurring in the Natron
Lakes is the reverse of what occurs
in the laboratory.
CaCO3 + 2NaCl → Na2CO3 + CaCl2
Using this insight
Berthollet reasoned that the reaction
is reversible, and that the relative amounts of “reactants” and “products” determine the direction in which
the reaction occurs, and the final composition of the reaction
mixture. We recog- nize a reaction’s ability
to move in both directions by using a double arrow
when writing the reaction.
Na2CO3 + CaCl2 < = = = =
> 2NaCl + CaCO3
Berthollet’s reasoning that reactions are reversible was an important step in understanding chemical reactivity. When we mix together solutions of Na2CO3
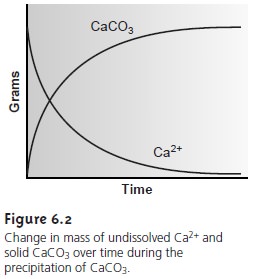
and CaCl2, they react to produce NaCl and CaCO3. If we monitor the mass of
dissolved Ca2+ remaining and the mass of CaCO3 produced as a function
of time, the result will look something like the graph in Figure 6.2. At the start of the reaction the mass of dissolved Ca2+ decreases
and the mass of CaCO3 in- creases.
Eventually, however, the reaction
reaches a point after which no further changes
occur in the amounts of these species.
Such a condition
is called a state of equilibrium.
Although a system
at equilibrium appears
static on a macroscopic level,
it is important to remember that the forward
and reverse reactions still occur. A reac-
tion at equilibrium exists in a “steady
state,” in which
the rate at which any species
forms equals the rate at which it is consumed.
Related Topics