Chapter: Modern Analytical Chemistry: Equilibrium Chemistry
Solving Equilibrium Problems: pH of a Polyprotic Acid or Base
pH of a Polyprotic Acid or Base
A more challenging problem is to find the pH of a solution
prepared from a polyprotic acid or one of its conjugate species.
As an example, we will use the amino acid alanine whose
structure and acid dissociation constants are shown in Figure 6.11.
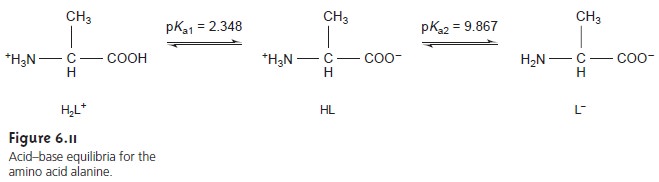
pH of 0.10 M H2L+
Alanine hydrochloride is a salt consisting of the diprotic
weak acid H2L+ and Cl–. Because H2L+ has two acid dissociation reactions, a complete systematic solution
to this problem will be more complicated than that for a mono- protic weak acid. Using a ladder diagram (Figure
6.12) can help us simplify
the problem. Since the areas of predominance for H2L+ and L– are widely separated, we can assume that any solution
containing an appreciable quantity of H2L+ will con- tain
essentially no L–. In this case, HL is such a weak acid that H2L+ behaves as if it were
a monoprotic weak acid.

To find the
pH of 0.10 M H2L+, we assume that
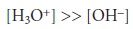
Because H2L+ is a relatively strong
weak acid, we cannot simplify
the problem fur- ther, leaving us with
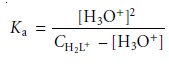
Solving the resulting quadratic equation gives
the [H3O+] as 1.91 x 10–2 M or a pH of 1.72. Our
assumption that [H3O+] is significantly greater
than [OH–] is acceptable.
pH of 0.10 M L–
The alaninate ion is a diprotic weak base, but using the ladder dia- gram as a guide
shows us that
we can treat
it as if it were
a monoprotic weak
base. Following the steps
in Example 6.11 (which is left as an exercise), we find that the
pH of 0.10 M alaninate is 11.42.
pH of 0.1 M HL
Finding the
pH of a solution of alanine is more complicated than that for H2L+
or L– because we must consider two equilibrium reactions involving HL. Alanine is an amphiprotic species, behaving as an acid
HL(aq)+ H2O(l) < = = = = > H3O+(aq)+ L–(aq)
and a base
HL(aq)+ H2O(l) <
=== ==
> OH–(aq)+ H2L+(aq)
As always, we must also consider the dissociation of water
2H2O(l) < == == > H3O+(aq)+ OH–(aq)
This leaves us with five unknowns ([H2L+], [HL], [L–], [H3O+], and [OH–]), for which we need five
equations. These equations are Ka2 and Kb2 for HL,
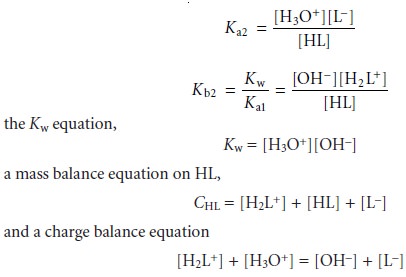
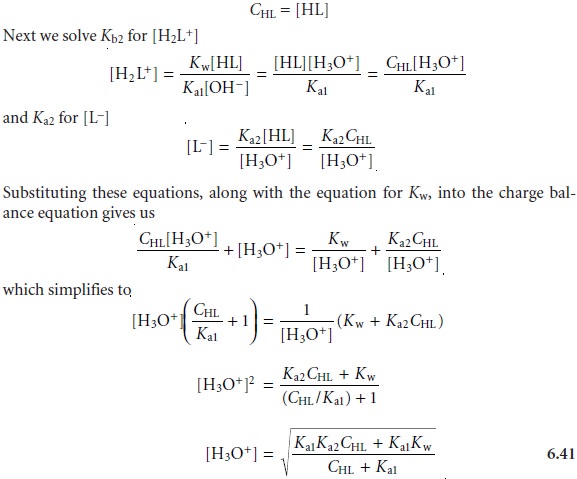
From the ladder
diagram it appears
that we may safely assume
that the concentra- tions of H2L+ and L– are significantly smaller than that
for HL, allowing us to sim- plify the mass balance
equation to
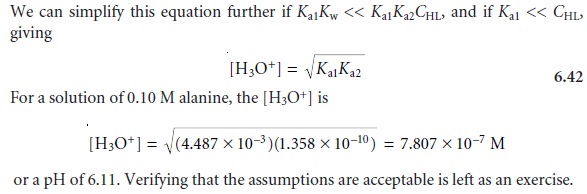
Triprotic Acids and Bases, and Beyond
The treatment of a diprotic
acid or base is
easily extended to acids and bases having
three or more acid–base sites.
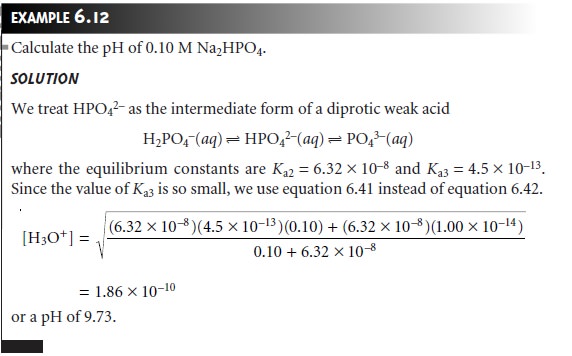
For a tripro- tic weak acid such as H3PO4, for example, we can treat
H3PO4 as if it was a mono- protic weak acid, H2PO4– and HPO 2– as if they were intermediate forms
of diprotic weak acids,
and PO 3– as if it was
a monoprotic weak
base.
Related Topics