Chapter: Modern Analytical Chemistry: Equilibrium Chemistry
Equilibrium Constants for Complexation Reactions
Complexation Reactions
A more general definition
of acids and bases was proposed by G. N. Lewis (1875–1946) in 1923. The Brønsted–Lowry definition of acids and bases focuses
on an acid’s proton-donating ability and a base’s proton-accepting ability. Lewis the- ory, on the other
hand, uses the breaking and forming of covalent bonds
to describe acid–base characteristics. In this treatment, an acid is an electron pair acceptor, and a
base is an electron pair
donor. Although Lewis
theory can be applied to the treat- ment of acid–base reactions, it
is more useful for treating complexation reactions between metal
ions and ligands.
The following reaction between the metal
ion Cd2+ and the ligand NH3 is typical of a complexation reaction.
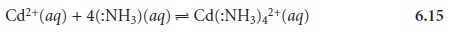
The product of this reaction is called a metal–ligand complex. In writing the
equa- tion for this reaction, we have shown
ammonia as :NH3 to emphasize the pair of electrons it donates to Cd2+. In subsequent reactions we will omit
this notation.
The formation of a metal–ligand complex is described by a formation con- stant, Kf. The
complexation reaction between
Cd2+ and NH3, for example, has the following
equilibrium constant
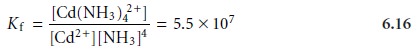
The reverse of reaction 6.15 is called
a dissociation reaction
and is characterized by a dissociation constant,
Kd, which
is the reciprocal of Kf.
Many complexation reactions occur in a stepwise fashion.
For example, the re-
action between Cd2+ and NH3 involves four successive reactions
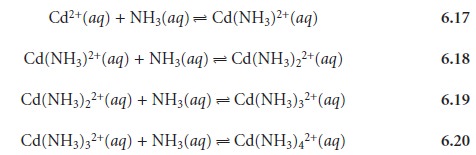
This creates a problem since it no longer is clear what reaction is described by a for- mation constant. To avoid ambiguity, formation
constants are divided
into two cat- egories. Stepwise formation
constants, which are designated as Ki for the ith step, describe the successive addition
of a ligand to the metal–ligand complex
formed in the previous
step. Thus, the equilibrium constants
for reactions 6.17–6.20
are, re- spectively, K1, K2, K3, and K4. Overall, or cumulative formation
constants, which are designated as βi, describe the addition
of i ligands to the free metal ion. The
equilibrium constant expression given in equation 6.16, therefore, is correctly iden- tified as β4, where
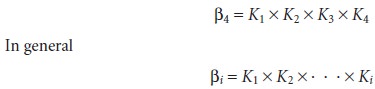
Stepwise and cumulative formation constants for selected metal–ligand complexes are given in Appendix 3C.
Equilibrium constants for complexation reactions
involving solids are defined
by combining appropriate Ksp and Kf expressions. For example, the solubility of AgCl
increases in the presence of excess chloride
as the result of the following com- plexation reaction
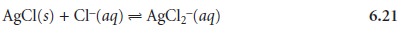
This reaction can
be separated into
three reactions for
which equilibrium constants are known—the solubility of AgCl, described by its Ksp
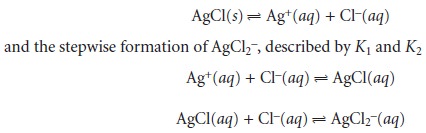
The equilibrium constant for reaction 6.21, therefore, is equal
to Ksp x K1 x K2
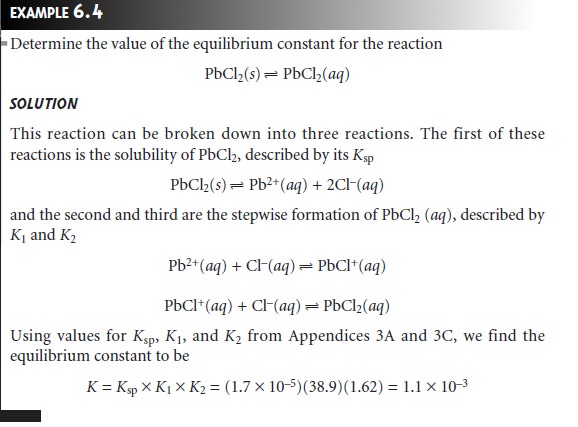
Related Topics