Chapter: 11th 12th std standard Class Physics sciense Higher secondary school College Notes
Pressure exerted by a gas
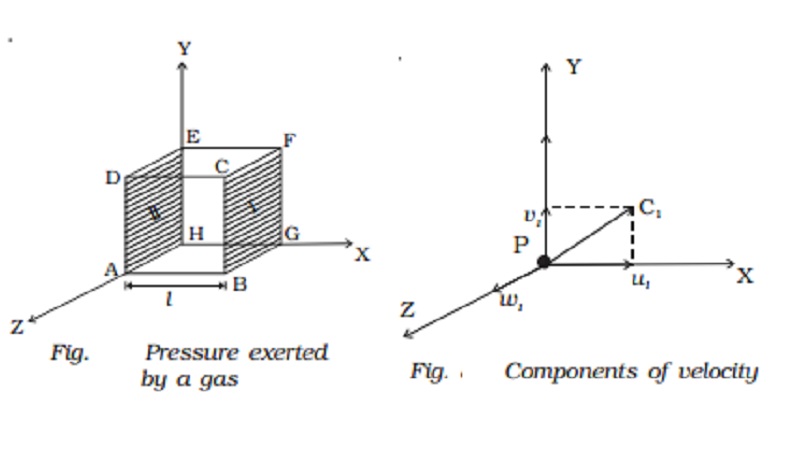
Pressure exerted by a gas
The molecules of a gas are in a
state of random motion. They continuously collide against the walls of the
container. During each collision, momentum is transfered to the walls of the
container. The pressure exerted by the gas is due to the continuous collision
of the molecules against the walls of the container.
Due to this continuous collision,
the walls experience a continuous
force which is equal to the total momentum imparted to the walls per second.
The force experienced per unit area of the walls of the container determines
the pressure exerted by the gas.
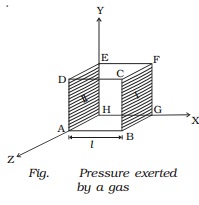
Consider a cubic container of
side l containing n molecules of perfect gas moving with velocities C1, C2, C3
... Cn (Fig.). A molecule moving with a velocity
C1, will have velocities u1, v1 and w1 as components along the x, y and z axes
respectively. Similarly u2, v2 and w2 are the velocity components of the second
molecule and so on. Let a molecule P (Fig.) having velocity C1 collide against the
wall marked I (BCFG) perpendicular to the x-axis. Only the x-component of the
velocity of the molecule is relevant for the wall I. Hence momentum of the
molecule before collision is mu1 where m is the mass of the molecule. Since the
collision is elastic, the molecule will rebound with the velocity u1 in the
opposite direction. Hence momentum of the molecule after collision is ?mu1.
Change in the momentum of the
molecule
= Final momentum - Initial
momentum
= ?mu1 ? mu1 = ?2mu1
During each successive collision
on face I the molecule must travel a distance 2l from face I to face II and
back to face I.
Time taken between two successive
collisions is = 2l/u1
∴ Rate of change of momentum = Change in the momentum / Time
taken
= (-2mu1) / (2l/u1)
= (-2mu12) / (2l)
= -mu12 / l
(i.e) Force exerted on
the molecule = -mu12
/ l
∴ According to Newton?s third law of motion, the force exerted by
the molecule
= -(-mu12
/ l) = mu12 / l
Force exerted by all
the n molecules is
Fx= mu12
/ l + mu22 / l + ?? + mun2 / l
Pressure exerted by
the molecules
Px=Fx/A
=1/l2 ( [mu12]/l
+ [mu22]/l +
?..+ [mun2]/l )
=m/l3(u12
+ u22 + ?.+ un2 )
Similarly, pressure
exerted by the molecules along Y and Z axes are
Py = m/l3(v12+
v22+ ???+ vn2)
Px = m/l3(w12+
w 22+ ???+ w n2)
Since the gas exerts
the same pressure on all the walls of the container
Px = Py
= Pz = P
P = [Px + Py
+ Pz ]/3
P = 1/3 . m/l3. [C12
+ C22 + .... + Cn2 ]
where C12
= (u12 + v12 + w12
)
P = 1/3 . mn/V . C2
where C is called the
root mean square (RMS) velocity, which is defined as the square root of the
mean value of the squares of velocities of individual molecules.
(i.e.) C = root[ (C12
+ C22 +?.+ Cn2 ) / n ]
Relation between the pressure exerted by a gas and the mean
kinetic energy of
translation per unit volume of the gas
Pressure exerted by
unit volume of a gas, P =
mnC2 / 3
P = 1/3 . ρC2 (∵ mn = mass per unit
volume of the gas ; mn = ρ, density of the gas)
Mean kinetic energy of
translation per unit volume of the gas
E =1/2 . ρC2
P/E = 2/3
P=2/3 . E
Average kinetic energy per molecule of the gas
Let us consider one
mole of gas of mass M and volume V
P = 1/3 . ρC2
P = 1/3 . M/V . C2
PV = 1/3 . M . C2
From gas equation
PV = RT
∴ RT =1/3 . M C2
3/2 . RT = ? . M C2
(i.e) Average kinetic
energy of one mole of the gas is equal to 3/2 . RT
Since one mole of the
gas contains N number of atoms where N is the Avogadro number
we have M = Nm
(1/2 ) . mNC2
= 3/2 . RT
? mC2 = 3/2
. R/N . T
=3/2 kT where k =R/N, is
the Boltzmann constant Its value is 1.38 ? 10-23 J K-1
∴ Average kinetic energy per molecule of the gas is equal to 3/2
kT
Hence, it is clear
that the temperature of a gas is the measure of the mean translational kinetic
energy per molecule of the gas.
Related Topics