Chapter: 11th 12th std standard Class Physics sciense Higher secondary school College Notes
Degrees of freedom of molecule
Degrees of freedom
The number of degrees of freedom of a dynamical
system is defined as the total number of co-ordinates or independent variables
required to describe the position and configuration of the system.
For translatory motion
(i) A particle moving in a straight
line along any one of the axes has one degree of freedom (e.g) Bob of an
oscillating simple pendulum.
(ii) A particle moving in a plane (X
and Y axes) has two degrees of freedom. (eg) An ant that moves on a floor.
(iii)
A particle moving in space (X, Y and Z axes) has three degrees
of freedom. (eg) a bird that flies.
A point mass cannot undergo rotation, but only translatory motion. A rigid body with finite mass has both rotatory and translatory motion. The rotatory motion also can have three co-ordinates in space, like translatory motion ;
Therefore a rigid body will
have six degrees of freedom ; three due to translatory motion and three due to
rotatory motion.
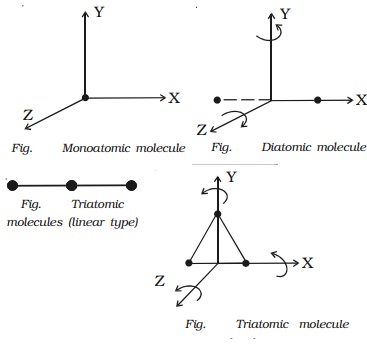
1 Monoatomic molecule
Since a monoatomic molecule
consists of only a single atom of point mass it has three degrees of freedom of
translatory motion along the three co-ordinate axes as shown in Fig..
Examples : molecules
of rare gases like helium, argon, etc.
2 Diatomic molecule
The diatomic molecule
can rotate about any axis at right angles to its own axis. Hence it has two
degrees of freedom of rotational motion in addition to three degrees of freedom
of translational motion along the three axes. So, a diatomic molecule has five degrees
of freedom (Fig.). Examples : molecules of O2, N2, CO, Cl
2, etc.
3 Triatomic molecule (Linear type)
In the case of
triatomic molecule of linear type, the centre of mass lies at the central atom.
It, therefore, behaves like a diamotic molecule with three degrees of freedom
of translation and two degrees of freedom of rotation, totally it has five
degrees of freedom (Fig.). Examples : molecules of CO2, CS2,
etc.
4 Triatomic molecule (Non-linear type)
A triatomic non-linear
molecule may rotate, about the three mutually perpendicular axes, as shown in
Fig.. Therefore, it possesses three degrees of freedom of rotation in addition
to three degrees of freedom of translation along the three co-ordinate axes. Hence
it has six degrees of freedom. Examples : molecules of H2O, SO2, etc.
In all the above
cases, only the translatory and rotatory motion of the molecules have been
considered. The vibratory motion of the molecules has not been taken into
consideration.
Related Topics