Chapter: Psychology: The Brain and the Nervous System
Plasticity
PLASTICITY
Overall, then, it should be clear
that the brain—indeed, the entire nervous system— contains many distinct
regions. Each region has its own job to do, and so we can under-stand the
nervous system only by keeping track of the various parts—where each is, what
it does, and how each contributes to a person’s overall functioning.
But there’s one more layer of
complexity that we need to confront, because the functioning and arrangement of
the brain can change during our
lifetimes. Neurons can change their pattern of connections—so that, in effect,
the brain gets new “wiring.” And there is increasing evidence that the brain
can grow new neurons in certain circumstances. These changes are fascinating on
their own—but they also hold out a promise of enormous importance: Perhaps we
can harness this potential for change in order to repair the brain when it has been damaged through injury or
disease. Let’s look at the nature of brain
plasticity—the brain’s capacity to alter its structure and function.
Changes in Neuronal Connections
Each day brings us new
experiences, and through them we learn new facts, acquire new skills, and gain
new perspectives. Our reactions to the world—indeed, our entire
personalities—evolve as we acquire knowledge, maturity, and maybe even wisdom.
These various changes all correspond to changes in the nervous system, making
it clear that the nervous system must somehow be plastic—subject to alteration.
In fact,
the nervous system’s
plasticity takes many
different forms. Among
other options, individual neurons can alter their “output”—that is, can
change the amount of neurotransmitter
they release. On the “input” side, neurons can also change how sensitive they are to neurotransmitters
by literally gaining new receptors. Both of these alterations play
a pivotal role
in learning, and
we’ll return to
these mechanisms.
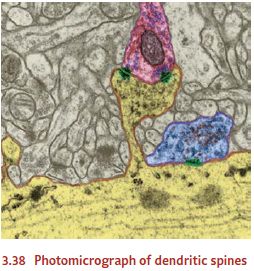
Neurons can also create entirely new connections, producing new synapse in
response to new patterns of stimulation. The changes in this case seem to take
place largely on the dendrites of postsynaptic cells. The dendrites grow ne
dendritic spines—little knobs attached to the surface of the dendrites (Figure
3.38; Kolb, Gibb, & Robinson, 2003; Moser, 1999; Woolf, 1998). These spines
are the “receiving stations” for most synapses; so
growing more spines
almost certainly means
that, as learning proceeds, the
neuron is gaining new synapses—new points of communication with its cellular
neighbors.
Cortical Reorganization
Plasticity in the nervous system
can also lead to larger-scale changes in the brain’s archi-tecture. In one
study, investigators trained monkeys to respond in one way if they heard a
certain musical pitch and in another way if they heard a slightly different
pitch (Recanzone, Schreiner, & Merzenich, 1993). We know from other
evidence that—just as in humans—the monkeys’ projection areas for sounds are
organized in maps; different sites on the monkey’s cortex are responsive to
different frequencies of sound. After train-ing, though, the map of the
monkey’s auditory projection area was reorganized, so that much more cortical
area was now devoted to the frequencies emphasized during training.
Can the same plasticity be
demonstrated in humans? One research team used neu-roimaging to examine the
somatosensory projection areas in a group of highly trained musicians, all of
whom played string instruments; a comparison group consisted of nonmusicians
(Elbert, Pantev, Wienbruch, Rockstroh, & Taub, 1995). The results showed
that in the musicians’ brains, more cortical area was dedicated to the
represen-tation of input from the fingers—suggesting that because of their
instrumental training, the musicians’ brains had been reorganized to devote
more tissue to skills essential for their playing.
A related result comes from a
study of London cabdrivers. These drivers need sophis-ticated navigation skills
to find their way around London, and they become more and more skillful as they
gain experience. This skill, in turn, is reflected directly in their brain
structure: Studies show that these cabdrivers have enlarged hippocampi—and the
hippocampus, you’ll recall, is a brain structure crucial for navigation.
Further, the more years of cab-driving experience an individual had, the
greater the degree of hip-pocampal enlargement (E. Maguire et al., 2000).
Even more evidence comes from
research with the blind. In one study, investigators used neuroimaging to
compare the brain activity in blind and sighted research partici-pants who were
exploring a surface with their fingertips (Sadato et al., 1996). The sighted
participants showed the expected pattern of increased activity in
somato-sensory areas as they felt the target surface. In contrast, during this
task the blind participants showed increased activity in the visual cortex. Apparently, for these
individ-uals, this brain area had taken on a new job. No longer occupied with
visual informa-tion, this area of cortex had shifted to the entirely new task
of processing information from the fingertips (also see Kauffman et al., 2002).
Thus it seems that the brain is
plastic both at the microscopic level, where it involves changes in how neurons
communicate with each other, and at a much grander level. If a person receives
a lot of practice in a task, more brain tissue is recruited for the task—presumably
because the tissue has been “reassigned” from some other task. Likewise,
sensory cortex that was initially sensitive to one modality can apparently be
reassigned to an entirely different modality.
New Neurons
The last form of plasticity we’ll
look at has been controversial because a long-held doctrine in neuroscience was
that, at birth, the brain has all the neurons it will ever have. As a result,
plasticity during the organism’s lifetime must be due to changes in these
neurons. However, neuroscientists have been expressing reservations about this
doctrine for years (e.g., Ramón y Cajal, 1913), and it turns out that those
reservations were justified. There is now clear evidence that new neurons
continue to develop throughout an organism’s lifetime and that this growth is
promoted by learning and enriched experience (Eriksson et al., 1998).
The evidence suggests, however,
that neurogenesis—the birth of new
neurons—is very slow in the adult human brain; and it seems that most of these
new neurons don’t survive for long (Scharfman & Hen, 2007; Shors, 2009).
It’s also unclear whether neu-rogenesis occurs in all parts of the adult
brain—and, in particular, whether it occurs in the cerebral cortex (Bhardwaj et
al. 2006). If it doesn’t, this may be a regard in which humans are different
from many other species.
In some ways, these results seem
backwards. One would think that the creation of new neurons would allow
flexibility for the organism and so would contribute to learning— and therefore
would be most prominent in species (including humans!) that are capable of
especially sophisticated learning. Yet it seems that we may be the species for
which cor-tical neurogenesis is least
likely. What explains this pattern? One hypothesis is that human intellectual
capacities depend on our being able to accumulate
knowledge, building on things we have already learned. This in turn may require
some degree of biological stabil-ity in the brain, so that we do not lose the
skills and knowledge we’ve already acquired. For this purpose, we may need a
permanent population of cortical neurons—and this means not introducing new neurons.
From this perspective, the absence of neuronal growthmight limit our
flexibility; but it might nonetheless be a good thing, helping to sustain long-term
retention of complex knowledge (Rakic, 2006; Scharfman & Hen, 2007).
Repairing Damage to the Nervous System
Notice, then, that plasticity has
its advantages and disadvantages. On the positive side, plasticity makes it
possible to “rewire” the nervous system in response to new informa-tion and new
experience. On the negative side, plasticity may in some circumstances
undermine the stability of a pattern of neural connections and thus may be
disruptive. So perhaps it’s not surprising that different species have evolved
to have different degrees of plasticity, with the pattern presumably dependent
on that species’ need for flexibility or for longer-term retention.
There’s one arena, though, in
which plasticity is certainly desirable: If the nervous system is damaged
through injury or disease, the effects can be disastrous. It would be
wonderful, therefore, if the nervous system could repair itself by growing new
neurons or reestablishing new connections. This sort of self-repair is often
possible in the peripheral nervous system; there, neurons can regenerate their
axons even after the original axon has been severed. Unfortunately, this sort
of regrowth after damage seems not to occur in humans’ central nervous system,
and here, once nerve fibers are dam-aged, they generally stay damaged. (For
some of the mechanisms blocking this self-repair, see W.-Y.Kim & Snider,
2008.)
Is there some way, however, to
use the processes of plasticity observed in healthy brains to restore damaged
brains? If so, we might be able to repair the damage created by injury—such as
the spinal injury suf-fered by actor Christopher Reeve, well known for his role
in the Superman movies (Figure 3.39).
After his injury, Reeve spent the rest of his life paralyzed but devoted his
energy and talent to encouraging research on spinal cord injuries and other
nerve damage. A means of restoring damaged brains might also give hope to those
suffering from Alzheimer’s dis-ease or Parkinson’s disease, both of which
involve the destruc-tion of brain tissue (Figure 3.40).
Researchers are actively
exploring these issues; some of their efforts are focused on encouraging the
growth of new neurons (e.g., W.-Y. Kim & Snider, 2008), and other research


is seeking to implant new tissue
into the brain in order to replace the damaged cells. Some of the most exciting
work, however, is in a third category and involves a mix of implanting tissue
and encouraging growth. Specifically, this effort involves implanting, into an
area of damage, the same sorts of stem
cells that are responsible for building the nervous system in the first
place. Stem cells are, in general, cells that are found in early stages of an
organ-ism’s development and that have not yet begun to specialize or
differentiate in any way. The idea in using these cells is that we would not be
replacing the damaged brain tissue directly. Instead, the stem cells would,
once in place, serve as precursors of the cells the brain needs—so the brain
could, in effect, grow its own replacements.
Preliminary studies in animals
suggest that when stem cells are injected into a patch of neurons, the cells
are—as we would hope—induced to turn into healthy neurons just like their
neighbors, taking the same shape, producing the same neurotransmitters, and
filling in for dead neurons (Holm et al., 2001; Isacson, 1999; Philips et al.,
2001; Sawamoto et al., 2001). Thus it seems plausible that stem-cell therapy may
provide a means of treatment for various forms of brain injury—and so far the
results have been quite encouraging (Kondziolka et al., 2000; Veizovic, Beech,
Stroemer, Watson, & Hodges, 2001). In one remarkable study, researchers
inserted human stem cells into the damaged spines of laboratory rats. The rats,
which had been paralyzed at the study’s start, recovered well enough so that
they could walk again (Cizkova et al., 2007).
Research in this domain
continues. In early 2009, the U.S. Food and Drug Administration gave permission
for the first clinical trials for stem-cell therapy in humans who had suffered
spinal cord injury. However, the progress of research in this arena has been
slow—largely due to an ethical debate over where the stem cells usually come
from. As part of a fertility treatment, a woman’s ova are sometimes removed,
fertilized in a labo-ratory, and allowed to develop into large masses of cells.
One of these masses is then placed back into the woman in hopes that it will
implant in the uterus and develop into a normal pregnancy. The other masses—the
ones not implanted—were for many years the main sources for stem cells, and
there lies the problem: In principle, these other masses might also have been
implanted and might also have developed into a human fetus. On that basis, some
people have argued that use of these cells for any other purpose is
essen-tially a form of abortion and thus is unacceptable to anyone who opposes
abortion.
This issue led President George
W. Bush to limit the use of federal research money for studies relying on
embryonic stem cells. Early in 2009, President Barack Obama reversed that
policy—so we can now expect the pace of research to accelerate. Meanwhile,
investigators are also seeking to develop alternative sources of stem cells;
this may allow us to avoid the ethical debate altogether. One way or another,
stem cell research is certain to continue; and in light of the evidence so far,
this research holds enormous promise for treating—and perhaps reversing—a range
of profoundly dis-abling diseases as well as helping people who have suffered
tragic injuries.
Related Topics