Chapter: 11th Chemistry : UNIT 13 : Hydrocarbons
Physical Properties of Alkanes
Physical Properties:
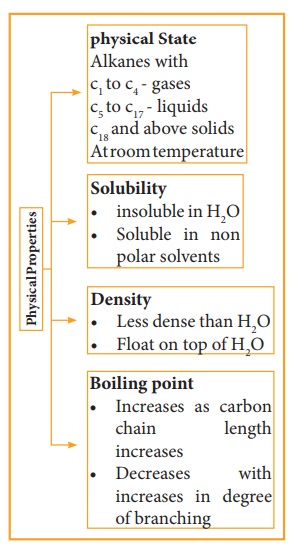
1) Boiling Point and Physical state
The boiling point of continuous chain alkanes increases with increases in length of carbon chain roughly about 30°C for every added carbon atom to the chain. Being non polar, alkanes have weak Vanderwal’s force which depends upon molecular surface area and hence increases with increase molecular size. We observe that with same number of carbon atoms, straight chain isomers have higher boiling point compared to branch chain isomers. The boiling point decreases with increase in branching as the molecule becomes compact and the area of the contact decreases.
2) Solubility and density
Water molecules are polar and alkanes are non-polar. The insolubility of alkanes in water makes them good water repellent for metals which protects the metal surface from corrosion. Because of their lower density than water, they form two layers and occupy top layer. The density difference between alkanes and water explains why oil spills in aqueous environment spread so quickly.
Related Topics