Chapter: 11th Chemistry : UNIT 13 : Hydrocarbons
Conformations of alkane(ethane)
Conformations of alkane:
Each carbon in alkanes is sp3 hybridized and the four groups or atoms around the carbon are tetrahedrally bonded. In alkanes having two or more carbons, there exists free rotation about C-C single bond. Such rotation leaves all the groups or atoms bonded to each carbon into an infinite number of readily interconvertible three dimensional arrangements. Such readily interconvertible three dimensional arrangement of a molecule is called conformations.
(i) Conformations of ethane:
The two tetrahedral methyl groups can rotate about the carbon – carbon bond axis yielding several arrangements called conformers. The extreme conformations are staggered and eclipsed conformation. There can be number of other arrangements between staggered and eclipsed forms and their arrangements are known as skew forms.
Eclipsed conformation:
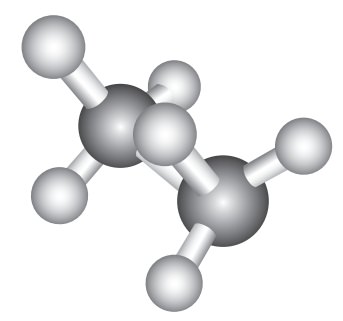
In this conformation, the hydrogen’s of one carbon are directly behind those of the other. The repulsion between the atoms is maximum and it is the least stable conformer.
Staggered conformation:
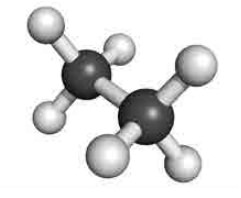
In this conformation, the hydrogens of both the carbon atoms are far apart from each other. The repulsion between the atoms is minimum and it is the most stable conformer.
Skew Conformation :
The infinite numbers of possible intermediate conformations between the two extreme conformations are referred as skew conformations.
The stabilities of various conformations of ethane are
Staggered > Skew > Eclipsed
The potential energy difference between the staggered and eclipsed conformation of ethane is around 12.5 KJmol-1. The various conformations can be represented by new man projection formula.
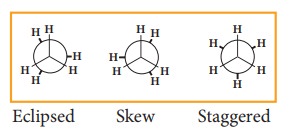
projection formula for Ethane
Conformations of n-Butane:
n-Butane may be considered as a derivative of ethane, as one hydrogen on each carbon is replaced by a methyl group
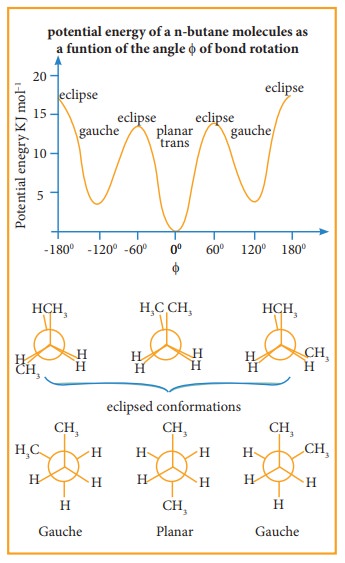
Eclipsed conformation:
In this conformation, the distance between the two methyl group is minimum. So there is maximum repulsion between them and it is the least stable conformer.
Anti or staggered form
In this conformation, the distance between the two methyl groups is maximum and so there is minimum repulsion between them. And it is the most stable conformer.
The following potentially energy diagram shows the relative stabilities of various conformers of n-butane.
Related Topics