Hydrocarbons | Chemistry - Nomenclature and isomerism of Alkanes | 11th Chemistry : UNIT 13 : Hydrocarbons
Chapter: 11th Chemistry : UNIT 13 : Hydrocarbons
Nomenclature and isomerism of Alkanes
Nomenclature and isomerism:
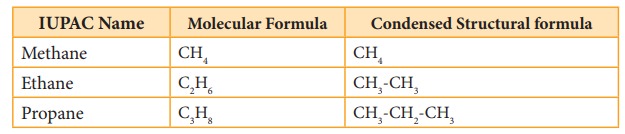
We have already discussed the nomenclature of organic compound in Unit:11. Let us understand the nomenclature and isomerism in few examples. The first three members methane CH4, ethane C2H6 and propane C3H8 have only one structure.
However, higher members can have more than one structure leading to constitutional isomers (differ in connectivity) or structural isomers. For example, an alkane with molecular formula C4H10 can have two structures. They are n-butane and iso-butane. In n-butane, all the four carbon atoms are arranged in a continuous chain. The ‘n’ in n-butane stand for ‘normal’ and means that the carbon chain is unbranched. The second isomer iso-butane has a branched carbon chain. The word iso indicates it is an isomer of butane.
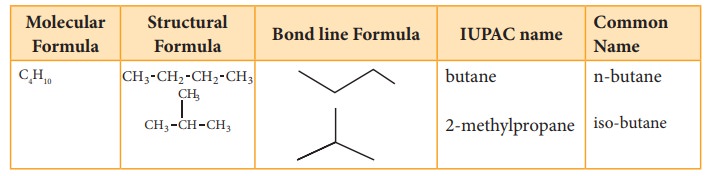
Though both the structures have same molecular formula but their carbon chains differ leading to chain isomerism
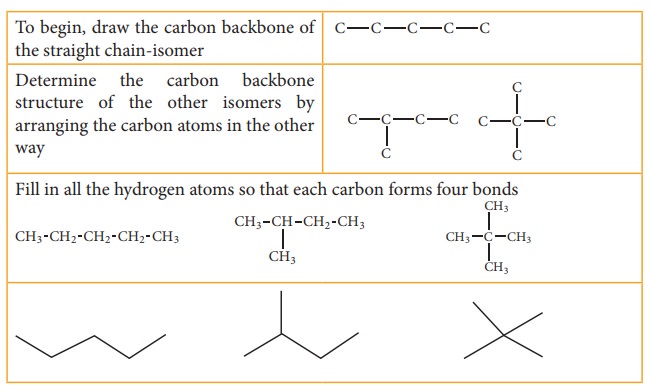
Let us understand the chain isomerism by writing the isomers of pentane C5H12
Solution:
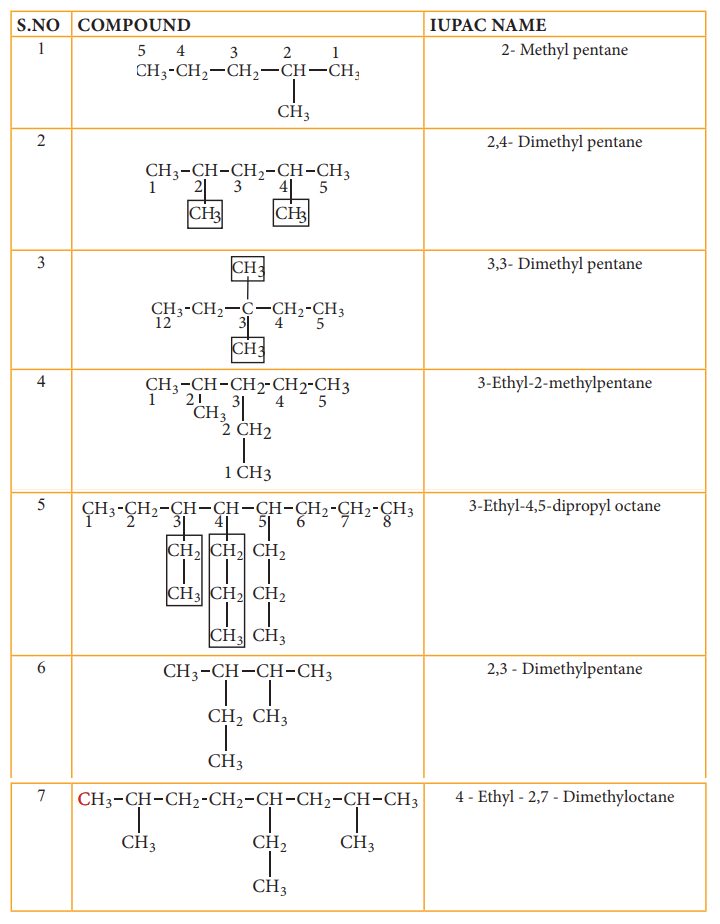
IUPAC name for some branched alkanes
Let us write the IUPAC name for the below mentioned alkanes by applying the general rules of nomenclature that we already discussed in unit No.11
How to draw structural formula for given IUPAC name :
After you learn the rules for naming alkanes, it is relatively easy to reverse the procedure and translate the name of an alkane into a structural formula. The example below show how this is done.
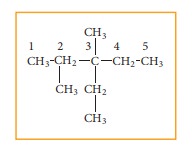
Let us draw the structural formula for
a) 3-ethyl-2,3-dimethyl pentane
Solution:
Step: 1The parent hydrocarbon is pentane. Draw the chain of five carbon atoms and number it.
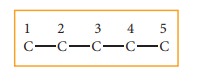
Step :2 Complete the carbon skeleton by attaching the alkyl group as they are speci-fied in the name. An ethyl group is attached to carbon 3 and two methyl groups are attached to carbon 2 and 3.
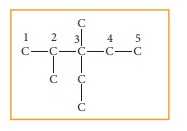
Step: 3 Add hydrogen atoms to the carbon skeleton so that each carbon atoms has four bonds
Related Topics