Chapter: 11th Chemistry : UNIT 13 : Hydrocarbons
Chemical properties of Alkanes
Chemical properties:
Alkanes are quite unreactive towards most reagents. However under favorable conditions, alkanes undergo the following type of reaction.
Paraffin is the older name for the alkane group family of compounds. This name comes from the Latin which means ‘little activity’
1) Combustion:
A combustion reaction is a chemical reaction between a substances and oxygen with evolution of heat and light (usually as a flame). In the presence of sufficient oxygen, alkanes undergoes combustion when ignited and produces carbondioxide and water.
The combustion reaction is expressed as follows
for example:
CH4 +2O2 → CO2 + 2H2O ΔH°=-890.4kJ
When alkanes burn in insufficient supply of oxygen, they form carbonmonoxide and carbon black.
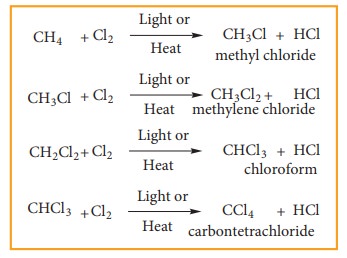
2) Halogenation:
Ahalogenation reaction is the chemical reaction between an alkane and halogen in which one or more hydrogen atoms are substituted by the halogens.
Chlorination and Bromination are two widely used halogenation reactions. Fluorination is too quick and iodination is too slow. Methane reacts with chlorine in the presence of light or when heated as follows.
Mechanism:
The reaction proceeds through the free radical chain mechanism. This mechanism is characterized by three steps initiation, propagation and termination.
i) CHAIN INITITATION: The chain is initiated by UV light leading to homolytic fission of chlorine molecules into free radicals (chlorine atoms).
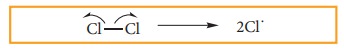
Here we choose Cl-Cl bond for fission because C-C & C-H bonds are stronger than Cl-Cl.
ii) PROPAGATION: It proceeds as follows,
a) Chlorine free radial attacks the methane molecule and breaks the C-H bond resulting in the generation of methyl free radical
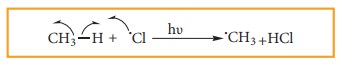
b) The methyl free radical thus obtained attacks the second molecule of chlorine to give chloromethane (CH3Cl) and a chlorine free radical as follows.
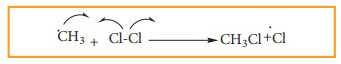
c) This chlorine free radical then cycles back to step (a) and both step (a) and (b) are repeated many times and thus chain of reaction is set up.
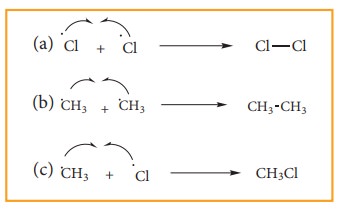
iii) Chain termination:
After sometimes, the reactions stops due to consumption of reactant and the chain is terminated by the combination of free radicals.
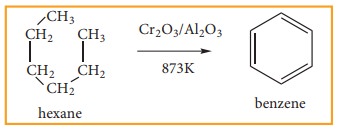
3) Aromatisation
Alkanes with six to ten carbon atoms are converted into homologous of benzene at high temperature and in the presence of catalyst. This process is known as aromatization. It occurs by simultaneous cyclisation followed by dehydrogenation of alkanes.
n-Hexane passed over Cr2O3 supported on alumina at 873 K gives benzene.
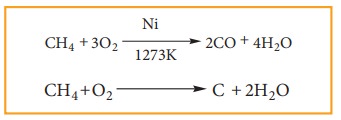
4) Reaction With Steam:
Methane reacts with steam at 1273K in the presence of Nickel and decomposes to form carbon monoxide and hydrogen gas.
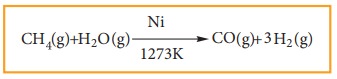
Production of H2 gas from methane is known as steam reforming process and it is a well-established industrial process for the production of H2 gas from hydrocarbons.
5) Pyrolysis
Pyrolysis is defined as the thermal decomposition of organic compound into smaller fragments in the absence of air through the application of heat. ‘Pyro’ means ‘fire’ and ‘lysis’ means ‘separating’. Pyrolysis of alkanes also named as cracking.
In the absence of air, when alkane vapours are passed through red-hot metal it breaks down into simpler hydrocarbons.
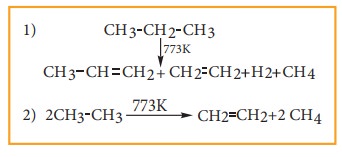
The products depends upon the nature of alkane, temperature, pressure and presence or absence of catalyst. The ease of cracking in alkanes increases with increase in molecular weight and branching in alkanes. Cracking plays an important role in petroleum industry.
6) Isomerisation:
Isomerisation is a chemical process by which a compound is transformed into any its isomeric forms. Normal alkanes can be converted into branched alkanes in the presence of AlCl3 and HCl at 298 k.
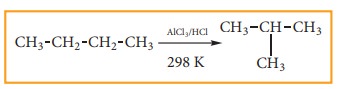
This process is of great industrial importance. The quality of gasoline is improved by isomerising its components.
Uses
The exothermic nature of alkane combustion reaction explains the extensive use of alkanes as fuels. Methane present in natural gas is used in home heating. Mixture of propane and butane are known as LPG gas which is used for domestic cooking purpose. GASOLINE is a complex mixture of many hydrocarbons used as a fuel for internal- combustion engines.
Carbon black is used in the manufacture of ink, printer ink and black pigments. It is also used as fillers.
Related Topics