Nomenclature and isomerism, Preparation, Physical and Chemical properties, Mechanism, Uses, IUPAC name, structural formula | Hydrocarbons | Chemistry - Alkenes | 11th Chemistry : UNIT 13 : Hydrocarbons
Chapter: 11th Chemistry : UNIT 13 : Hydrocarbons
Alkenes
Alkenes:
Alkenes
are unsaturated hydrocarbons that contain carbon-carbon double bond. They are
represented by the general formulae CnH2n where ‘n’
stands for number of carbon atoms in the molecule. Alkenes are also known as
olefins (in Latin - oil maker) because the first member ethene combines with
chlorine gas to form an oily liquid as a product.
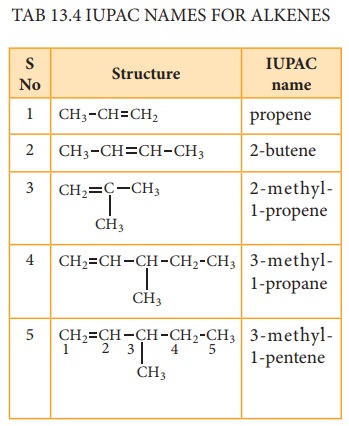
(i) Nomenclature Of Alkenes:
Let us write the IUPAC name for the
be-low mentioned alkanes by applying the general rules of nomenclature that we
already discussed in unit No.11
(ii)Isomerism:
![]()
![]() Presence of double bond in Alkene provides the possibility
of both structural and geometrical isomerism.
Presence of double bond in Alkene provides the possibility
of both structural and geometrical isomerism.
Structural Isomerism:
The
first two member’s ethene C2H4 and propene C3H6
do not have isomers because the carbon atoms in the molecules can be arranged
only one distinct way. However from the third member of alkene family butene C4H10,
structural isomerism exists.
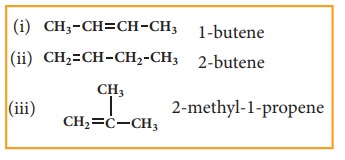
structures
(i) & (ii) are position isomers. structures (i) & (iii), (ii) &
(iii) are chain isomers.
Geometrical isomerism:
It
is a type of stereoisomerism and it is also called cis-trans isomerism. Such
type of isomerism results due to the restricted rotation of doubly bounded
carbon atoms.
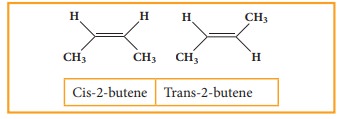
If
the similar groups lie on the same side, then the geometrical isomers are
called Cis-isomers. When the similar groups lie on the opposite side, it is
called a Trans isomer.
for
example: the geometrical isomers of 2-Butane is expressed as follows
General methods of preparation of alkenes:
(1) Preparation of alkene by dehydra-tion of alcohol:
When
an alcohol is heated at 430-440 K with excess of concentrated sulphuric acid, a
molecule of water from alcohol is removed and an alkene is formed. This
reaction is called elimination reaction.
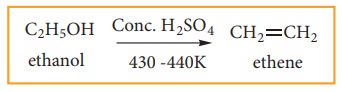
Ethene
can also be prepared in laboratory by catalytic dehydration of alcohol.
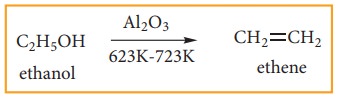
(2) Preparation of alkenes from alkynes:
Alkynes
can be reduced to cis-alkenes using Lindlar’s catalyst (CaCO3
sup-ported in palladuium partially deactivated with sulphur (or) gasoline).
This reaction is stero specific giving only the cis- alkene.
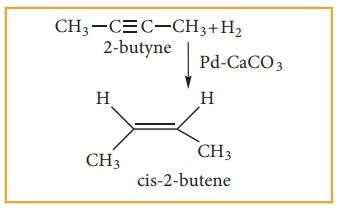
Alkynes
can also be reduced to trans-alkenes using sodium in liquid ammonia. This
reaction is stereospecific giving only the trans-alkene.
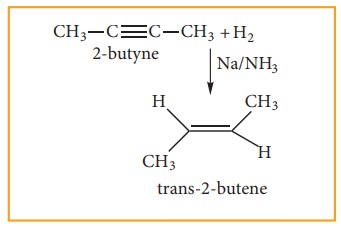
(3) Preparation of alkenes by dehydro-halogenaton of halo alkanes.
Halo
alkanes react with alcoholic KOH and eliminate hydrohalide resulting in the
formation of alkene.
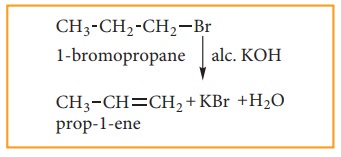
(4) Preparation of alkenes from vicinal dihalogen derivative of alkanes or vici-nal dihalides
The
compound in which two halogen atoms are attached to adjacent carbon-atoms are
called as vicinal dihalides. When vicinal dihalides are warmed with granulated
zinc in methanol, they lose a molecule of ZnX2 to form an alkene.
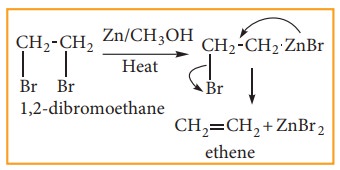
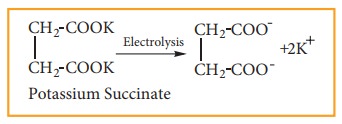
(5) Preparation of ethene by kolbe’s electrolytic method:
When
an aqueous solution of potas-sium succinate is electrolyzed between two
platinum electrodes, ethene is produced at the anode.
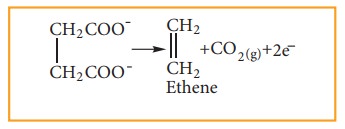
At
anode
Physical properities of alkenes:
The
first three members (Ethene, Propene and Butene) are gases, next fourteen
members are liquids and the higher alkenes are waxy solids. They are all
colourless and odourless except ethene which has a sweet smell.
1.
The melting and boiling point of alkenes increases along the homologous series.
Like alkanes, straight chain alkenes have high boiling point compared to its
isomeric branched alkenes.
2.
Alkenes are slightly soluble in water but readily in organic solvents.
Chemical properties of alkenes:
Alkenes
are more reactive than alkanes due to the presence of a double bond. The σ-
bond is strong but the π- bond is weak. The typical reactions of alkenes
involve addition of an electrophile across the double bonds proceeding through
ionic mechanism. However addition reactions proceed through free-radical
mechanism. Ozonolysis and polymerization are some of the characteristic
reactions of alkenes.
1. Addition Reactions
(i) Addition of hydrogen: (Hydroge-nation of alkenes)
hydrogen
adds on to alkenes in the presence of a metal catalyst (Ni, Pd (or) Pt) to
yield corresponding alkanes. This is known as catalytic hydrogenation. This process is of great importance in the manufacture of vanaspathi from
vegetable oil. This helps to prevent rancidity of vegetable oils.
(ii) Addition of halogens: (Halogena-tion of alkenes)
When
alkene is treated with halogens like chlorine or bromine, addition takes place
rapidly and forms 1, 2- dihalo alkane (or) vicinal dihalide.
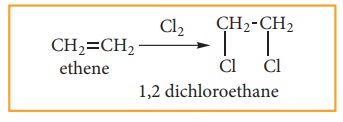
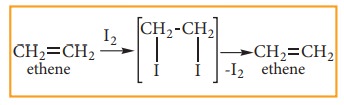
Iodine
reacts very slowly to form 1, 2 – diiodo alkane which are unstable and
re-generate the original alkene by elimination of iodine.
TEST
FOR ALKENE:

Bromine
in water is reddish brown colour. When small about of bromine water is added to
an alkene, the solution is decolourised as it forms dibromo compound. So, this
is the characteristic test for unsaturated compounds.
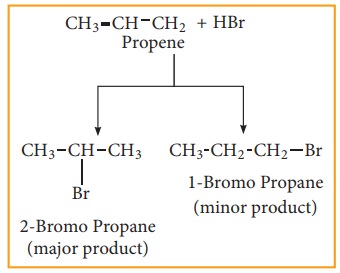
Markovnikoff ’s rule:
![]()
![]() “When an unsymmetrical alkene reacts with hydrogen halide,
the hydrogen adds to the carbon that has more number of hydrogen and halogen
add to the car-bon having fewer hydrogen”. This rule can also be stated as in
the addition reaction of alkene / alkyne, the most electro negative part of the
reagent adds on to the least hy-drogen attached doubly bonded carbon.
“When an unsymmetrical alkene reacts with hydrogen halide,
the hydrogen adds to the carbon that has more number of hydrogen and halogen
add to the car-bon having fewer hydrogen”. This rule can also be stated as in
the addition reaction of alkene / alkyne, the most electro negative part of the
reagent adds on to the least hy-drogen attached doubly bonded carbon.
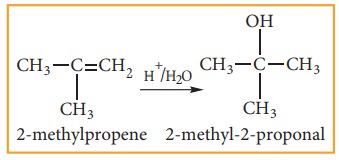
(iii) Addition of water:- (Hydration of alkenes)
Normally,
water does not react with alkenes. In the presence of concentrated sulphuric
acid, alkenes react with water to form alcohols. This reaction follows
carbo-cation mechanism and Markovnikoff ’s rule.
(iv) Addition of hydrohalides: (Hydro-halogenation of Alkenes)
Hydrogen
halides (HCl, HBr and HI) add to alkene to yield alkyl halides. The order of
reactivity of different hydrogen ha-lides is HI>HBr>HCl. It is an example
for electrophilic addition.
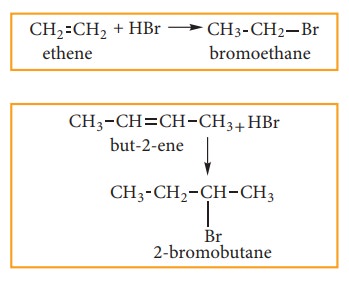
(a) Addition of HBr to symmetrical alkene:
Addition
of HBr to symmetrical alkene (similar groups are attached to dou-ble bond)
yields alkyl halides (haloalkanes)
(b) Addition HBr to unsymmetrical alkene:
In
the addition of hydrogen halide to an unsymmetrical alkene, two products are
obtained.
Mechanism:
Consider
addition of HBr to propene
Step: 1 Formation of
electrophile:
In
H-Br, Br is more electronegative than H. When bonded electron moves to-ward Br,
polarity is developed and creates an electrophile H+ which attaches
the double bond to form carbocation, as shown below.
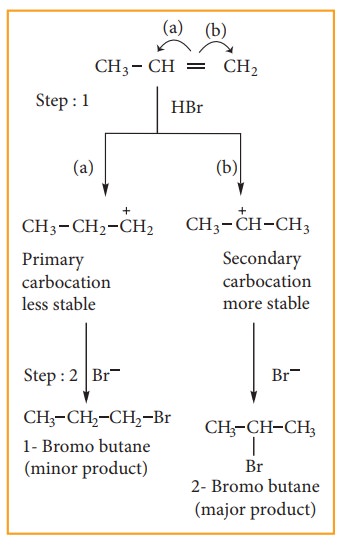
Step:2 Secondary carbocation is
more stable than primary carbocation and it
predominates over a the primary carbocation.
Step:3 The Br–ion attack the 2°
carbocation to from 2-Bromobutane,
the major product.
Consider
addition of HBr to 3-methyl-1-butene. Here the expected product according to
markovnikoff ’s rule is 2-bromo-3-methyl butane but the actual major product is
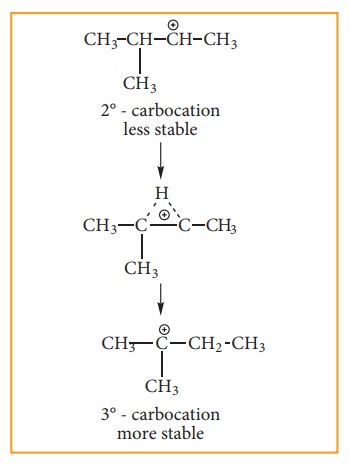
2-Bromo-2-methyl butane. This is because, the secondary carbocation formed
during the reaction rearranged to more stable tertiary carbocation. Attack of
Br– on this tertiary carbocation gives the major product 2-bromo-2-methyl
butane.
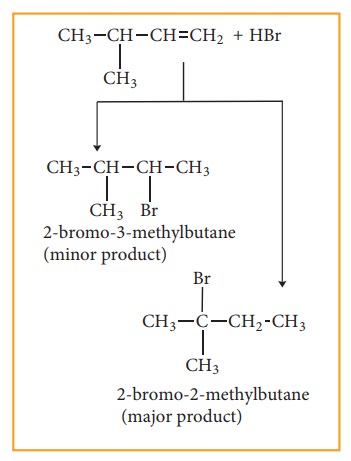
Carbocation
rearrangement
Anti-Markovnikoff ’s Rule (Or) Peroxide Effect (Or) Kharasch Addition
The
addition of HBr to an alkene in the presence of organic peroxide, gives the
anti Markovnikoff’s product. This effect is called peroxide effect.
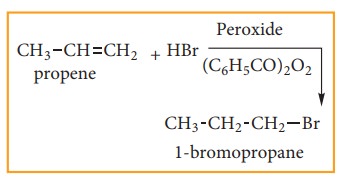
Mechanism:
The
reaction proceeds via free radical mechanism.
Step:1
The
weak O-O single bond linkages of peroxides undergoes homolytic cleavage to
generate free radical.
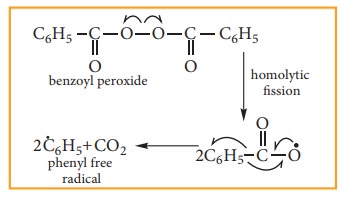
Step:2
The
radicals abstracts a hydrogen from HBr thus generating bromine radical.

Step:3
The
Bromine radical adds to the dou-ble bond in the way to form more stable al-kyl
free radical.
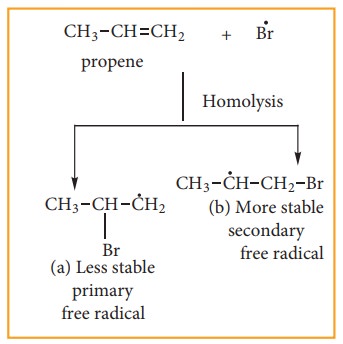
Step:4
Addition
of HBr to secondary free Radical
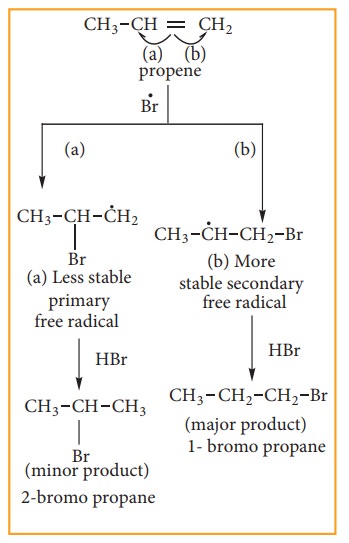
The
H-Cl bond is stronger (430.5 kJmol-1) than H-Br bond (363.7 kJmol-1),
thus H-Cl is not cleaved by the free radical. The H-I bond is weaker (296.8 kJ
mol-1), than H-Cl bond. Thus H-I bond breaks easily but iodine free
radicals combine to form iodine molecules instead of adding to the double bond
and hence peroxide effect is not observed in HCl& HI.
Kharasch Addition
Metal
catalysed free radical addition of CXCl3 Compounds to alkene is
called Kharash addition reaction
(v) Addition of sulphuric acid to alkenes
Alkenes
react with cold and concentrated sulphuric acid to form alkyl hydrogen sulphate
accordance with Markownikoff ‘s rule. Further hydrolysis yields alcohol.
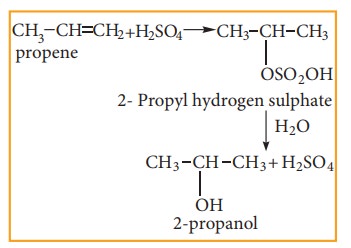
2. Oxidation:
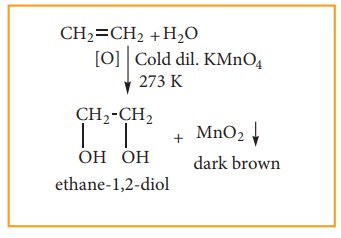
(i) With cold dilute alkaline KMnO4 solution (Baeyer’s Reagent)
Alkenes
react with Baeyer’s reagent to form vicinal diols. The purple solution (Mn2+)
becomes dark green (Mn6+), and then produces a dark brown
precipitate (Mn4+).
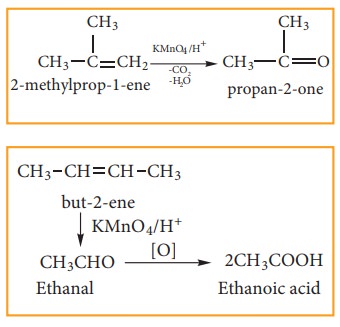
(ii) With acidified KMnO4 Solution:
Alkenes
react with acidified KMnO4 solution and are oxidised to ketones or
car-boxylic acid depends on the substituent at the olefinic carbon atom.. The
purple solu-tion becomes colourless. This is one of the test for unsaturation.
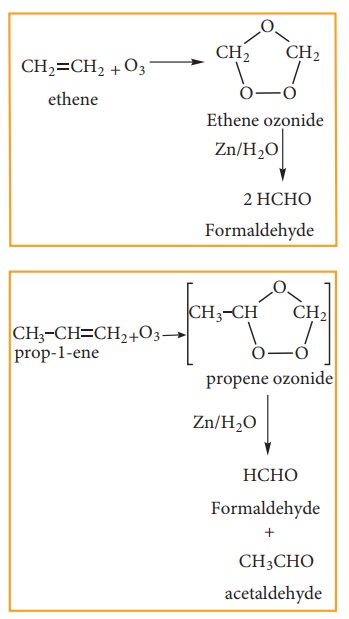
(iii) Ozonolysis:
Ozonolysis
is a method of oxidative cleavage of alkenes or alkynes using ozone and forms
two carbonyl compounds. Alkenes react with ozone to form Ozonide and it is
cleaved by Zn/H2O to form smaller molecules. This reaction is often
used to identify the structure of unknown alkene or alkyne by detecting the
position of double or triple bond.
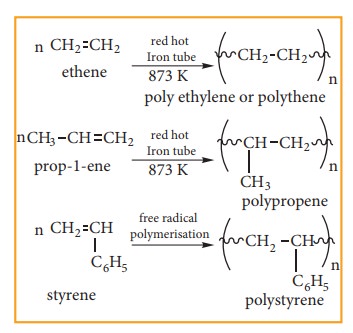
(iv) Polymerisation:
A
polymer is a large molecule formed by the combination of larger number of small
molecules. The process in known as polymerisation. Alkenes undergo
polymerisation at high temperature and pressure, in the presence of a catalyst.
for
example
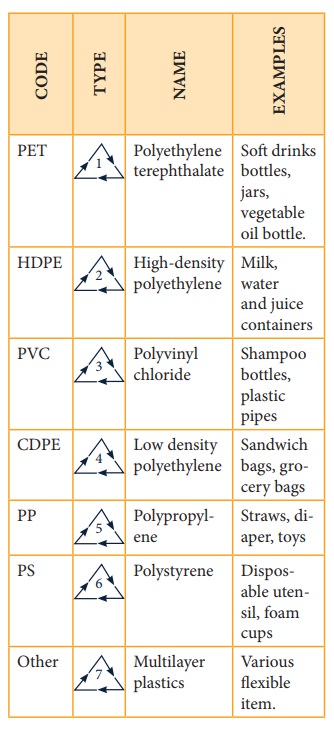
Recycling plastics
Extensive
use of polymers clogs up landfills and polute the environment. Be-cause of
diversity of polymers in consum-er products, recycling requires sorting the
polymers into various sub-types, labels with codes and symbols, which are then
recycled separately.
Table shows the codes and symbols used in recycling of ethene-based additionpolymers.
(Lower
the number, greater the ease of recycling the material)
Uses of Alkenes
1)
Alkenes find many diverse applications in industry. They are used as starting
materials in the synthesis of alcohols, plastics, liquors, detergents and fuels
2)
Ethene is the most important organic feed stock in the polymer industry. E.g.
PVC, Sarans and polyethylene. These polymer are used in the manufacture of
floor tiles, shoe soles, synthetic fibres, raincoats, pipes etc.,
Related Topics