Chapter: 11th Chemistry : UNIT 13 : Hydrocarbons
Chemical properties of alkenes
Chemical properties of alkenes:
Alkenes are more reactive than alkanes due to the presence of a double bond. The σ- bond is strong but the π- bond is weak. The typical reactions of alkenes involve addition of an electrophile across the double bonds proceeding through ionic mechanism. However addition reactions proceed through free-radical mechanism. Ozonolysis and polymerization are some of the characteristic reactions of alkenes.
1. Addition Reactions
(i) Addition of hydrogen: (Hydroge-nation of alkenes)
hydrogen adds on to alkenes in the presence of a metal catalyst (Ni, Pd (or) Pt) to yield corresponding alkanes. This is known as catalytic hydrogenation. This process is of great importance in the manufacture of vanaspathi from vegetable oil. This helps to prevent rancidity of vegetable oils.
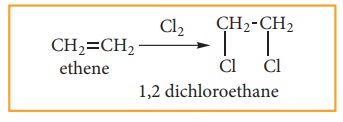
(ii) Addition of halogens: (Halogena-tion of alkenes)
When alkene is treated with halogens like chlorine or bromine, addition takes place rapidly and forms 1, 2- dihalo alkane (or) vicinal dihalide.
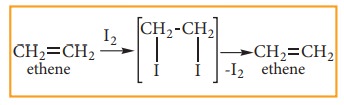
Iodine reacts very slowly to form 1, 2 – diiodo alkane which are unstable and re-generate the original alkene by elimination of iodine.
TEST FOR ALKENE:

Bromine in water is reddish brown colour. When small about of bromine water is added to an alkene, the solution is decolourised as it forms dibromo compound. So, this is the characteristic test for unsaturated compounds.
Markovnikoff ’s rule:
"When an unsymmetrical alkene reacts with hydrogen halide, the hydrogen adds to the carbon that has more number of hydrogen and halogen add to the car-bon having fewer hydrogen”. This rule can also be stated as in the addition reaction of alkene / alkyne, the most electro negative part of the reagent adds on to the least hy-drogen attached doubly bonded carbon.
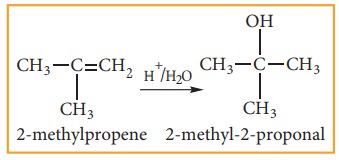
(iii) Addition of water:- (Hydration of alkenes)
Normally, water does not react with alkenes. In the presence of concentrated sulphuric acid, alkenes react with water to form alcohols. This reaction follows carbo-cation mechanism and Markovnikoff ’s rule.
(iv) Addition of hydrohalides: (Hydro-halogenation of Alkenes)
Hydrogen halides (HCl, HBr and HI) add to alkene to yield alkyl halides. The order of reactivity of different hydrogen ha-lides is HI>HBr>HCl. It is an example for electrophilic addition.
(a) Addition of HBr to symmetrical alkene:
Addition of HBr to symmetrical alkene (similar groups are attached to dou-ble bond) yields alkyl halides (haloalkanes)
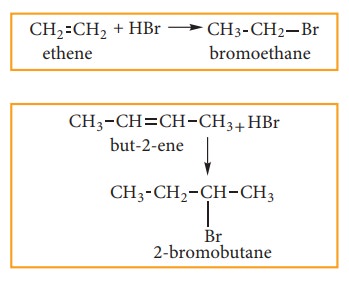
(b) Addition HBr to unsymmetrical alkene:
In the addition of hydrogen halide to an unsymmetrical alkene, two products are obtained.
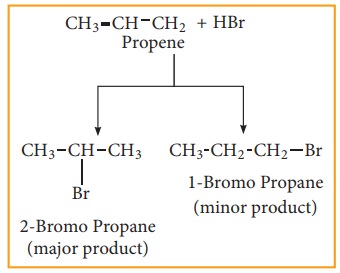
Mechanism:
Consider addition of HBr to propene
Step: 1 Formation of electrophile:
In H-Br, Br is more electronegative than H. When bonded electron moves to-ward Br, polarity is developed and creates an electrophile H+ which attaches the double bond to form carbocation, as shown below.
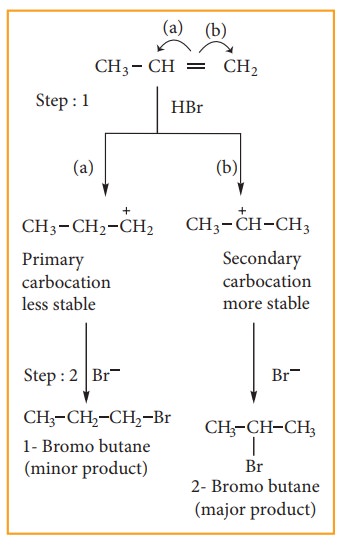
Step:2 Secondary carbocation is more stable than primary carbocation and it predominates over a the primary carbocation.
Step:3 The Br–ion attack the 2° carbocation to from 2-Bromobutane, the major product.
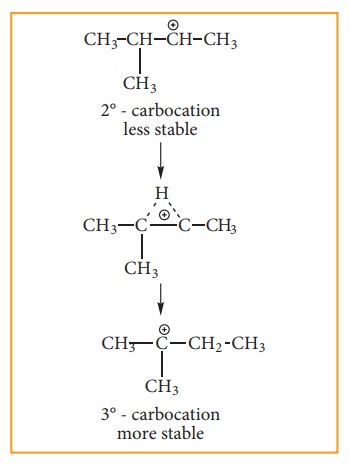
Consider addition of HBr to 3-methyl-1-butene. Here the expected product according to markovnikoff ’s rule is 2-bromo-3-methyl butane but the actual major product is 2-Bromo-2-methyl butane. This is because, the secondary carbocation formed during the reaction rearranged to more stable tertiary carbocation. Attack of Br– on this tertiary carbocation gives the major product 2-bromo-2-methyl butane.
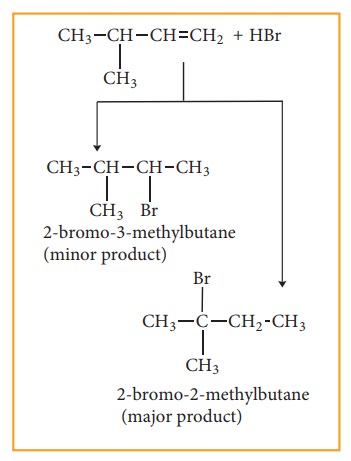
Carbocation rearrangement
Anti-Markovnikoff ’s Rule (Or) Peroxide Effect (Or) KharaschAddition
The addition of HBr to an alkene in the presence of organic peroxide, gives the anti Markovnikoff’s product. This effect is called peroxide effect.
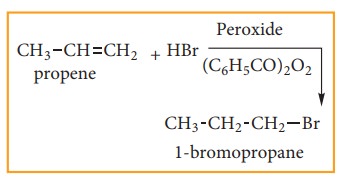
Mechanism:
The reaction proceeds via free radical mechanism.
Step:1
The weak O-O single bond linkages of peroxides undergoes homolytic cleavage to generate free radical.
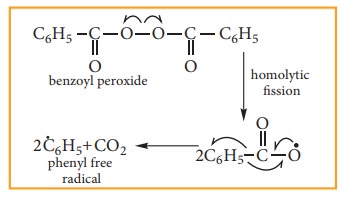
Step:2
The radicals abstracts a hydrogen from HBr thus generating bromine radical.

Step:3
The Bromine radical adds to the dou-ble bond in the way to form more stable al-kyl free radical.
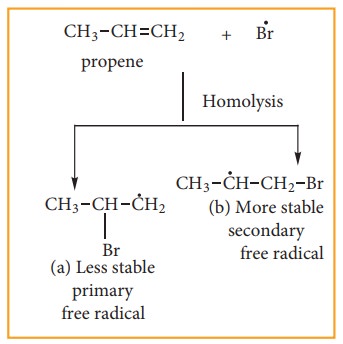
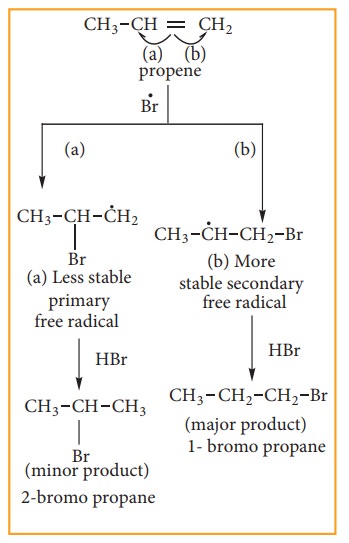
Step:4
Addition of HBr to secondary free Radical
The H-Cl bond is stronger (430.5 kJmol-1) than H-Br bond (363.7 kJmol-1), thus H-Cl is not cleaved by the free radical. The H-I bond is weaker (296.8 kJ mol-1), than H-Cl bond. Thus H-I bond breaks easily but iodine free radicals combine to form iodine molecules instead of adding to the double bond and hence peroxide effect is not observed in HCl& HI.
Kharasch Addition
Metal catalysed free radical addition of CXCl3 Compounds to alkene is called Kharash addition reaction
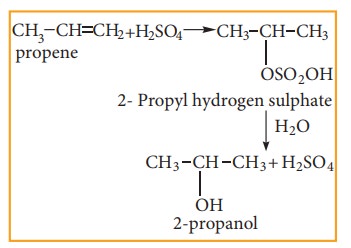
(v) Addition of sulphuric acid to alkenes
Alkenes react with cold and concentrated sulphuric acid to form alkyl hydrogen sulphate accordance with Markownikoff ‘s rule. Further hydrolysis yields alcohol.
2. Oxidation:
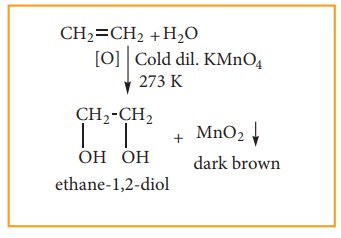
(i) With cold dilute alkaline KMnO4 solution (Baeyer’s Reagent)
Alkenes react with Baeyer’s reagent to form vicinal diols. The purple solution (Mn2+) becomes dark green (Mn6+), and then produces a dark brown precipitate (Mn4+).
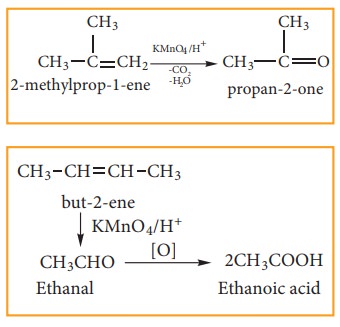
(ii) With acidified KMnO4 Solution:
Alkenes react with acidified KMnO4 solution and are oxidised to ketones or car-boxylic acid depends on the substituent at the olefinic carbon atom.. The purple solu-tion becomes colourless. This is one of the test for unsaturation.
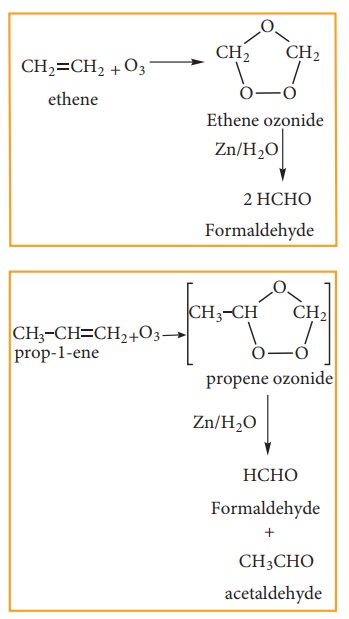
(iii) Ozonolysis:
Ozonolysis is a method of oxidative cleavage of alkenes or alkynes using ozone and forms two carbonyl compounds. Alkenes react with ozone to form Ozonide and it is cleaved by Zn/H2O to form smaller molecules. This reaction is often used to identify the structure of unknown alkene or alkyne by detecting the position of double or triple bond.
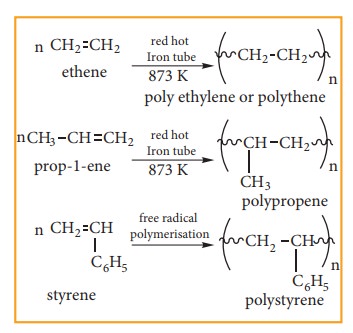
(iv) Polymerisation:
A polymer is a large molecule formed by the combination of larger number of small molecules. The process in known as polymerisation. Alkenes undergo polymerisation at high temperature and pressure, in the presence of a catalyst.
for example
Recycling plastics
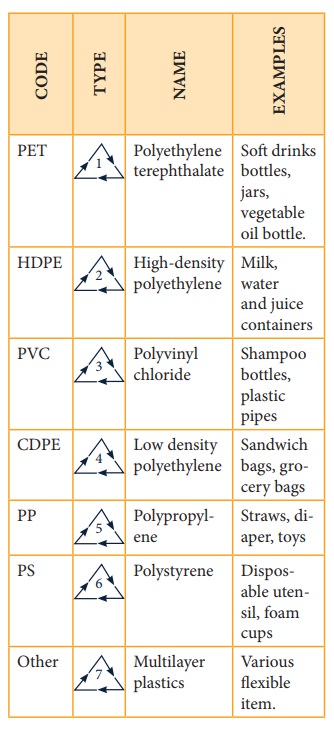
Extensive use of polymers clogs up landfills and polute the environment. Be-cause of diversity of polymers in consum-er products, recycling requires sorting the polymers into various sub-types, labels with codes and symbols, which are then recycled separately.
Table shows the codes and symbols used in recycling of ethene-based additionpolymers.
(Lower the number, greater the ease of recycling the material)
Related Topics