Chapter: 11th Chemistry : UNIT 13 : Hydrocarbons
Brief questions and answers: Chemistry: Hydrocarbons
Chemistry: Hydrocarbons
Answer the following questions
31. Give IUPAC names for the following compounds
i) CH3–CH=CH–CH=CH–C≡C–CH3
ii) 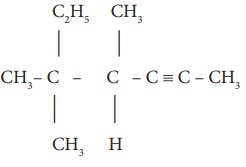
iii) (CH3)3 C – C ≡ C – CH (CH3)2
iv) ethyl isopropyl acetylene
v) CH ≡ C – C ≡ C – C ≡ CH
Answer:
1. Octa -
2,4- diene - 6 - yne
2. 5 ethyl-
4, 5, dimethyl hex - 2 - yne
3. 2, 2, 5
-Trimethylhex - 3 - yne
4. 2 -
Methylhex - 3 - yne
5. hexa −1,
3, 5 – triyne
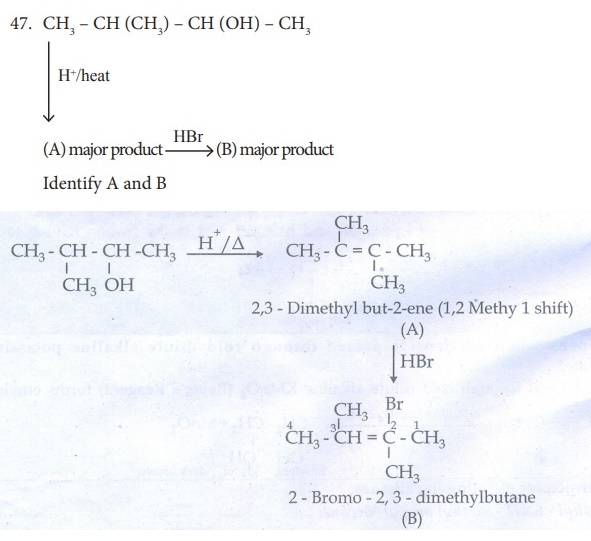
32. (i) Identify the compound A, B, C and D in the following series of reactions
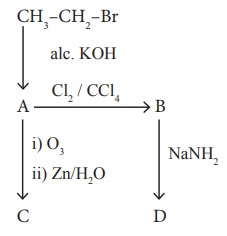
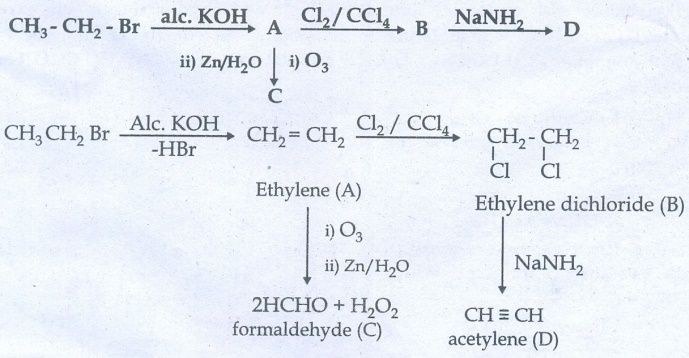
(ii) Write short notes on ortho, para directors in aromatic electrophilic substitution reactions.
●
Those group which increases electron density at ortho and para position are
known as ortho-para directors.
●
All the activating groups are ortho-para directors.
●
Example: -OH, -NH2-NHR, -NHCOCH3, -OCH3-CH3,
-C2H5 etc.
●
Let us consider the directive influences of phenolic (-OH) group. The resonance
hybrid of phenol.
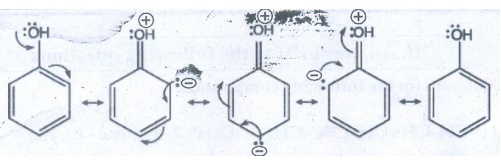
●
In the above resonance structures, the (−) charge is present on ortho and para
position of ring structure.
●
So, electron density is more at ortho and para position.
●
Therefore phenolic group activates the benzene ring for electrophilic attack at
ortho and para position and hence -OH group is ortho - para director and
activator.
●
Halogen group is an ortho-para director and deactivator eventhough halogen
group is strong − I effect which decreases the electron density of benzene
ring. However due to the presence of lone pair of electrons on halogen.
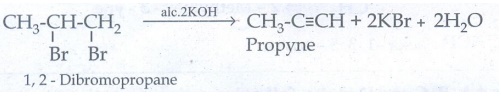
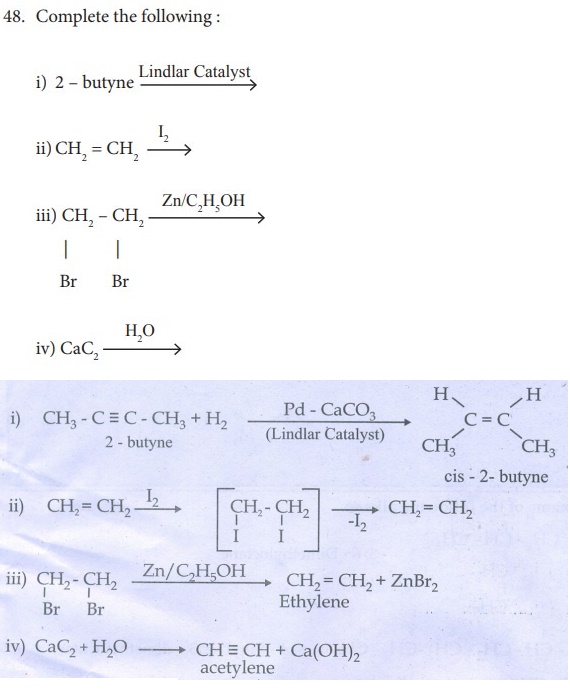
33. How is propyne prepared from an alkyene dihalide ?
34. An alkylhalide with molecular formula C6H13Br on dehydro halogenation gave two isomeric alkenes X and Y with molecular formula C6H12. On reductive ozonolysis, X and Y gave four compounds CH3COCH3, CH3CHO, CH3CH2CHO and (CH3)2 CHCHO. Find the alkylhalide.
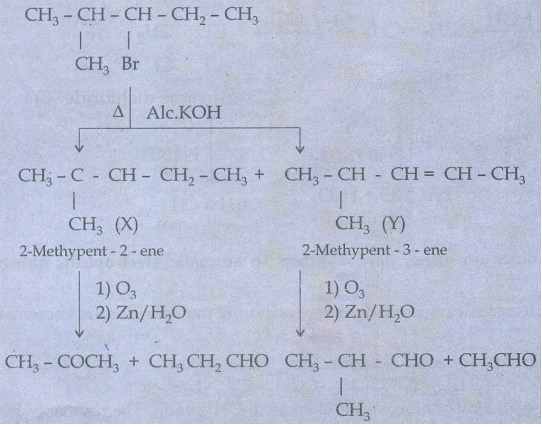
So, the alkyl halide is 3 Bromo - 2 methyl
pentane.
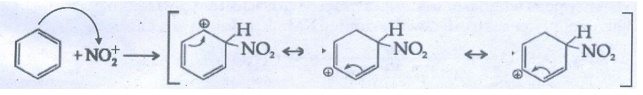
35. Describe the mechanism of Nitration of benzene.
Mechanism
of nitration of benzene:
●
Nitrating reagent: Cone. HNO3 + ConC. H2SO4
●
Electrophile : NO2 (nitronium ion)
●
Overall reaction :.

Step
1:
Generation of electrophile
HNO3
+ H2SO4 → NO2+ + HSO4+
+ H2O
Step
2: Attack
of NO2+ of on benzene to form carbocation which is
stabilized by resonance.
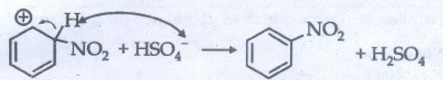
Step
3 : Loss of a proton from the carbocation in the presence of the base (HSO4−)
to form nitrobenzene.
36. How does Huckel rule help to decide the aromatic character of a compound.
Huckel
proposed that aromaticity is a function of electronic structure. A compound may
be aromatic. If it obeys the following rules.
i)
The molecule must be co-planar.
ii)
Complete delocalization of π electron in the ring.
iii)
Presence of (4n + 2) π electrons in the ring where n is an integer (n = 0, 1,
2).
This
is known as Huckel's rule.
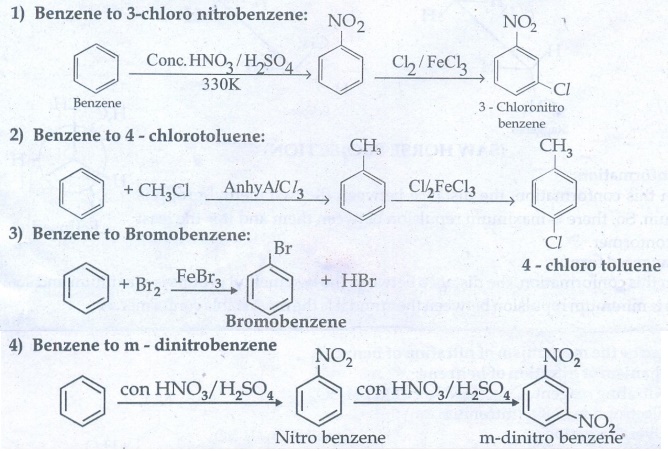
37. Suggest the route for the preparation of the following from benzene.
3 – chloro nitrobenzene
4 – chlorotoluene
Bromo benzene
m - dinitro benzene
1) Benzene to 3- chloro nitrobenzene :
2) Benzene to 4 – chlorotoluene :
3) Benzene to Bromobenzene:
4)
Benzene to m - dinitrobenzene
38. Suggest a simple chemical test to distinguish propane and propene.
1.
Propene will decolourise the brown colour of the solution of Br2/CCl4.
But propane will not decolourise the colour of bromine.
2.
Propene will decolourises the purple acidified KMnO4 solution, where
as propane does not.
39. What happens when isobutylene is treated with acidified potassium permanganate ?
When
isobutylene is treated with acidified KMnO4 solution gives acetone.

40. How will you convert ethyl chloride in to
i) ethane
ii) n – butane
(i)
Ethyle chloride to ethane:

(ii) Ethyl chloride to n-butane:
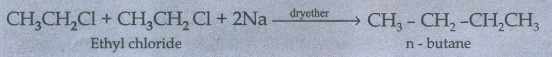
CH3CH2C1
Ethyl chloride + CH3CH2Cl
+ 2Na __ dryether __→ CH3 - CH2 -CH2CH3
n - butane
41. Describe the conformers of n - butane.
n-butane
may be considered as a derivatives of ethane, as one hydrogen on each carbon is
replaced by a methyl group.
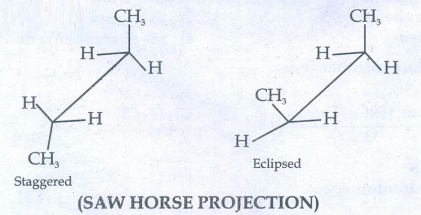
(SAW
HORSE PROJECTION)
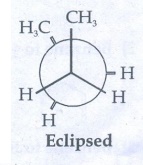
Eclipsed
conformation:
In
this conformation, the distance between the two methyl group is minimum. So,
there is maximum repulsion between them and it is the least stable conformer.
Anti
(or) Staggered form:
In
this conformation, the distance between the two methyl groups is maximum and so
there is minimum repulsion between them and it is the most stable conformer.
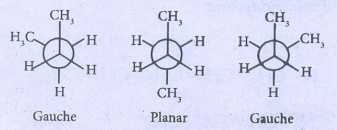
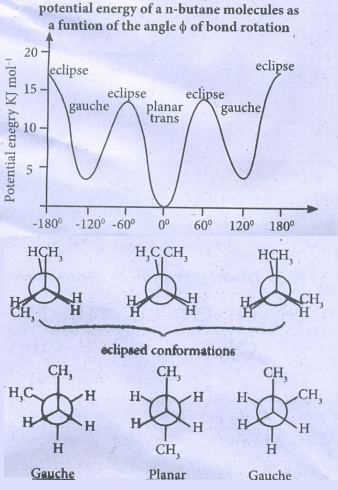
The
following potential energy diagram shows the stabilities of various conformers
of n-butane.
42. Write the chemical equations for combustion of propane.
The
combustion of propane is C3H8 + 5O2 → 3CO2+
4H2O
43. Explain Markow nikoff's rule with suitable example.
Markownikoff's
rule states:
"When
an unsymmetrical alkene reacts with hydrogen inninyyy the hydrogen
adds to the carbon that has more number of hydrogen and halogen add to the
carbon having fewer hydrogen"
Example:
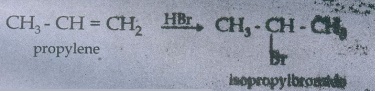
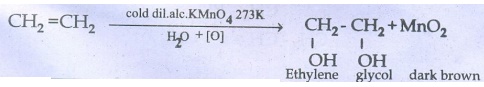
44. What happens when ethylene is passed through cold dilute alkaline potassium permanganate.
Ethylene
is passed through cold dilute alkaline KMnO4 (Bayer's Reagent) forms
ethylene glycol.
45. Write the structures of folowing alkanes.
i) 2, 3 – Dimethyl – 6 – (2 – methyl propyl) decane
ii) 5 – (2 – Ethyl butyl) – 3, 3 – dimethyldecane
iii) 5 – (1, 2 – Dimethyl propyl) – 2 – methylnonane

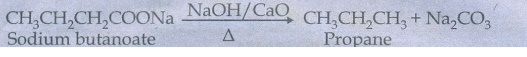
46. How will you prepare propane from a sodium salt of fatty acid ?
When
a mixture of sodium butanoate and soda lime is heated gives propane.
49 . How will you distinguish 1 – butyne and 2 – butyne?
1 - butyne is a terminal alkyne where as 2 - butyne is a non-terminal alkyne. Therefore, 1 - butyne gives white precipitate with Tollen's reagent and red precipitate with ammonical cuprous chloride while 2 - butyne will not give these test because of the absence of acidic hydrogen.
CH3 - CH2 - C ≡ CH + AgNO3 +
2NH4OH → CH3CH2C ≡ C - Ag ↓ + NH4NO3
+ H2O
But-l-yne : CH3 - CH2 - C ≡ CH
Silver butynide white ppt. : CH3CH2C ≡ C -
Ag ↓
CH3CH2 - C ≡ CH + Cu2Cl2
+ 2NH4OH → 2CH3CH2C ≡ C - Cu ↓ + 2NH4Cl
+ 2H2O
But - 1 - yne : CH3 - CH2 - C ≡ CH
Copper butynide : 2CH3CH2C ≡ C - Cu ↓
Related Topics