Chapter: 11th Chemistry : UNIT 13 : Hydrocarbons
Nomenclature and Isomerism of Alkenes
(i) Nomenclature Of Alkenes:
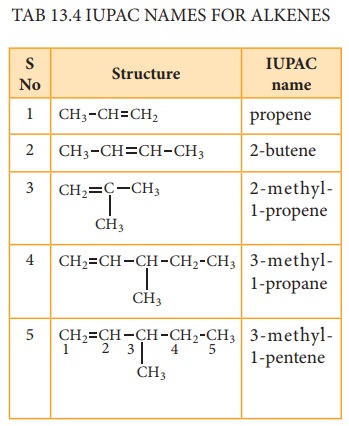
Let us write the IUPAC name for the be-low mentioned alkanes by applying the general rules of nomenclature that we already discussed in unit No.11
(ii) Isomerism:
![]()
![]()
Structural Isomerism:
The first two member’s ethene C2H4 and propene C3H6 do not have isomers because the carbon atoms in the molecules can be arranged only one distinct way. However from the third member of alkene family butene C4H10, structural isomerism exists.
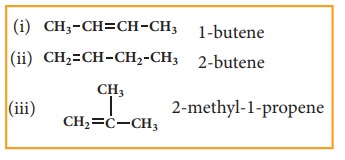
structures (i) & (ii) are position isomers. structures (i) & (iii), (ii) & (iii) are chain isomers.
Geometrical isomerism:
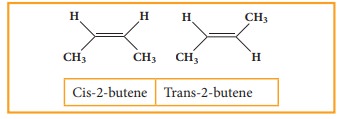
It is a type of stereoisomerism and it is also called cis-trans isomerism. Such type of isomerism results due to the restricted rotation of doubly bounded carbon atoms.
If the similar groups lie on the same side, then the geometrical isomers are called Cis-isomers. When the similar groups lie on the opposite side, it is called a Trans isomer.
for example: the geometrical isomers of 2-Butane is expressed as follows
Related Topics