Nomenclature and isomerism, Preparation, Physical and Chemical properties, Mechanism, Uses, IUPAC name, structural formula | Hydrocarbons | Chemistry - Alkanes | 11th Chemistry : UNIT 13 : Hydrocarbons
Chapter: 11th Chemistry : UNIT 13 : Hydrocarbons
Alkanes
Alkanes:
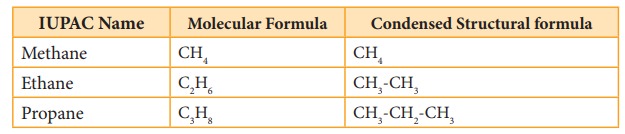
Alkanes are saturated hydrocarbons represented by the general formula CnH2n+2 where ‘n’ is the number of carbon atoms in the molecule. Methane CH4, is the first member of alkane family. The successive members are ethane C2H6, propane C3H8,butane C4H10, pentane C5H12 and so on. It is evident that each member differs from its proceeding or succeeding member by a –CH2 group.
Nomenclature and isomerism:
We
have already discussed the nomenclature of organic compound in Unit:11. Let us
understand the nomenclature and isomerism in few examples. The first three
members methane CH4, ethane C2H6 and propane C3H8
have only one structure.
However,
higher members can have more than one structure leading to constitutional
isomers (differ in connectivity) or structural isomers. For example, an alkane
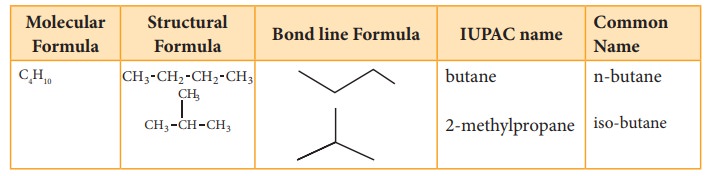
with molecular formula C4H10 can have two structures.
They are n-butane and iso-butane. In n-butane, all the four carbon atoms are
arranged in a continuous chain. The ‘n’ in n-butane stand for ‘normal’ and
means that the carbon chain is unbranched. The second isomer iso-butane has a
branched carbon chain. The word iso indicates it is an isomer of butane.
Though
both the structures have same molecular formula but their carbon chains differ
leading to chain isomerism
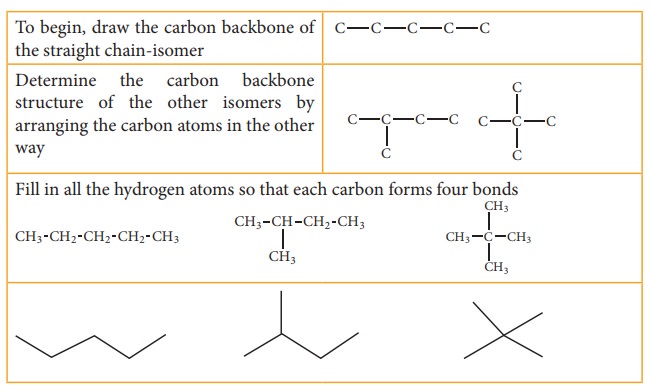
Let
us understand the chain isomerism by writing the isomers of pentane C5H12
Solution:
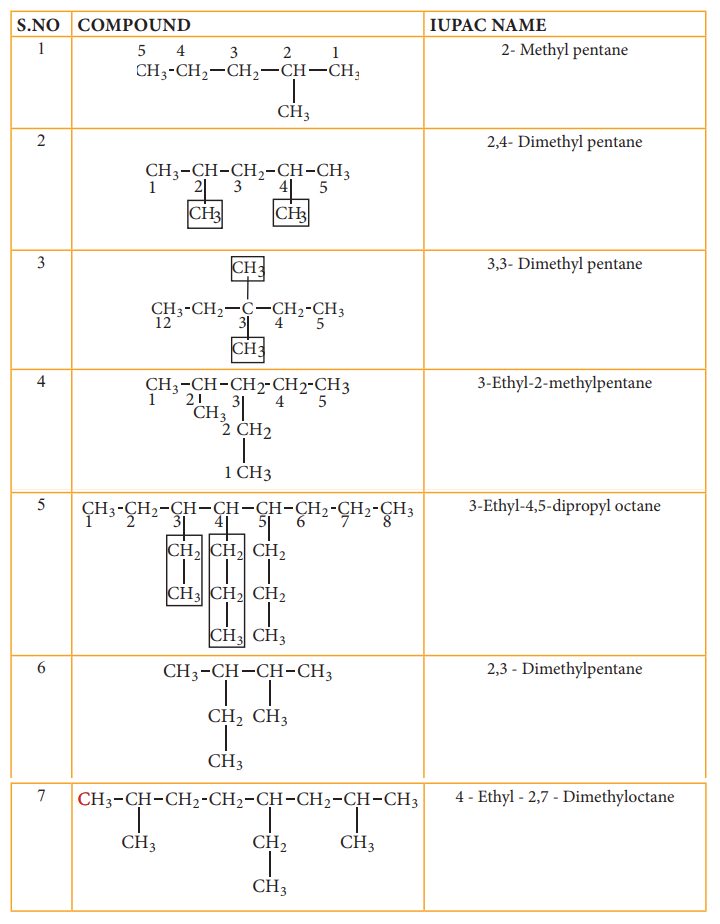
IUPAC name for some branched alkanes
Let us write the IUPAC name for the
below mentioned alkanes by applying the general rules of nomenclature that we
already discussed in unit No.11
How to draw structural formula for given IUPAC name :
After
you learn the rules for naming alkanes, it is relatively easy to reverse the
procedure and translate the name of an alkane into a structural formula. The
example below show how this is done.
Let
us draw the structural formula for
a)
3-ethyl-2,3-dimethyl pentane
Solution:
Step: 1The parent hydrocarbon
is pentane. Draw the chain of five
carbon atoms and number it.
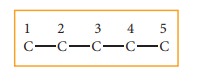
Step :2 Complete the carbon
skeleton by attaching the alkyl
group as they are speci-fied in the name. An ethyl group is attached to carbon
3 and two methyl groups are attached to carbon 2 and 3.
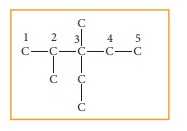
Step: 3 Add hydrogen atoms to
the carbon skeleton so that each carbon atoms has four
bonds
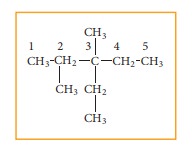
Preparation of alkanes:
Alkanes
are not laboratory curiosities but they are extremely important naturally
occurring compounds. Natural gas and petroleum (crude oil) are the most
important natural sources. However, it can be prepared by the following
methods.
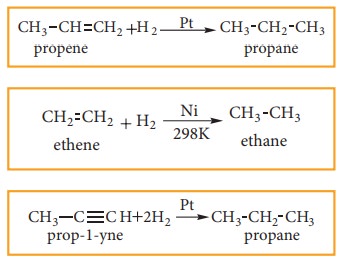
1. Preparation of alkanes from cata-lytic reduction of unsaturated hydro-carbons.
When
a mixture hydrogen gas with alkene or alkyne gas is passed over a catalysts
such as platinum or palladium at room temperature, an alkane is produced. This
process of addition of H2 to unsaturated compounds is known as
hydrogenationThe above process can be catalysed by nickel at 298K. This
reaction is known as Sabatier-Sendersens reaction
for
example:
2. Preparation of alkanes from carboxylic acids:
i) Decarboxylation of sodium salt of carboxylic acid
When a mixture of sodium salt of carboxylic acid and soda lime (sodium hydroxide + calcium oxide) is heated, alkane is formed. The alkane formed has one carbon atom less than carboxylic acid. This process of eliminating carboxylic group is known as decarboxylation.
for
example:
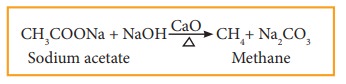
ii) Kolbe’s Electrolytic method
When
sodium or potassium salt of carboxylic acid is electrolyzed, a higher alkane is
formed. The decarboxylative dimerization of two carboxylic acid occurs. This
method is suitable for preparing symmetrical alkanes(R-R).
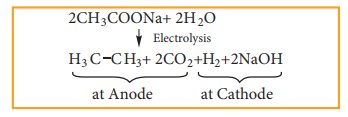
3.Preparation of alkanes using alkyl halides (or) halo alkanes
i) By reduction with nascent hydrogen
Except
alkyl fluorides, other alkyl halides can be converted to alkanes by reduction
with nascent hydrogen. The hydrogen for reduction may be obtained by using any
of the following reducing agents: Zn+HCl, Zn+CH3COOH, Zn-Cu couple
in ethanol, LiAlH4 etc.,
for
example:
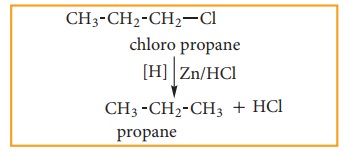
ii) Wurtz reaction
When
a solution of halo alkanes in dry ether is treated with sodium metal, higher alkanes are produced. This reaction is used to
prepare higher alkanes with even number of carbon atoms.
for
example:
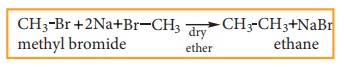
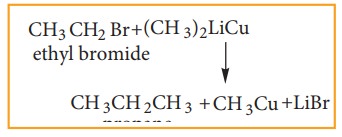
iii) Corey- House Mechanism
An
alkyl halide and lithium di alkyl cuprate are reacted to give higher alkane.
for
example:
4) Preparation of Alkanes from Gri-gnard reagents
Halo
alkanes reacts with magnesium in the presence of dry ethers to give alkyl
magnesium halide which is known as Grignard reagents. Here the alkyl group is
directly attached to the magnesium metal make it to behave as carbanion. So,
any compound with easily replaceable hydrogen reacts with Grignard reagent to
give corresponding alkanes.
for
example:
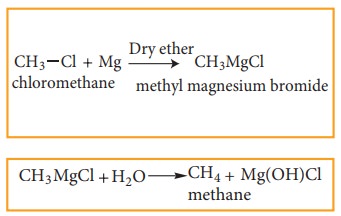
Physical Properties:
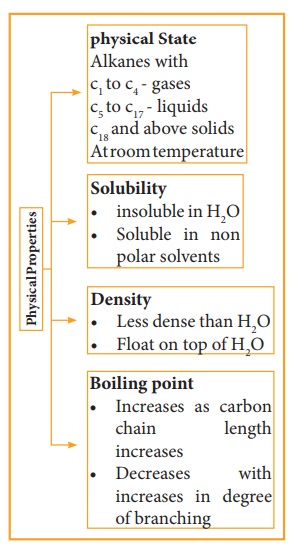
1) Boiling Point and Physical state
The
boiling point of continuous chain alkanes increases with increases in length of
carbon chain roughly about 30°C for every added carbon atom to the chain. Being
non polar, alkanes have weak Vanderwal’s force which depends upon molecular
surface area and hence increases with increase molecular size. We observe that
with same number of carbon atoms, straight chain isomers have higher boiling
point compared to branch chain isomers. The boiling point decreases with
increase in branching as the molecule becomes compact and the area of the
contact decreases.
2) Solubility and density
Water molecules are polar and alkanes are non-polar. The insolubility of alkanes in water makes them good water repellent for metals which protects the metal surface from corrosion. Because of their lower density than water, they form two layers and occupy top layer. The density difference between alkanes and water explains why oil spills in aqueous environment spread so quickly.
Conformations of alkane:
Each
carbon in alkanes is sp3 hybridized and the four groups or atoms around the
carbon are tetrahedrally bonded. In alkanes having two or more carbons, there
exists free rotation about C-C single bond. Such rotation leaves all the groups
or atoms bonded to each carbon into an infinite number of readily
interconvertible three dimensional arrangements. Such readily interconvertible
three dimensional arrangement of a molecule is called conformations.
(i) Conformations of ethane:
The
two tetrahedral methyl groups can rotate about the carbon – carbon bond axis
yielding several arrangements called conformers. The extreme conformations are
staggered and eclipsed conformation. There can be number of other arrangements
between staggered and eclipsed forms and their arrangements are known as skew
forms.
Eclipsed conformation:
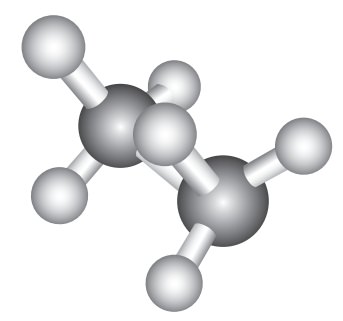
In
this conformation, the hydrogen’s of one carbon are directly behind those of
the other. The repulsion between the atoms is maximum and it is the least
stable conformer.
Staggered conformation:
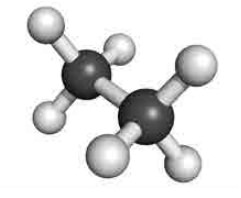
In
this conformation, the hydrogens of both the carbon atoms are far apart from
each other. The repulsion between the atoms is minimum and it is the most
stable conformer.
Skew Conformation :
The
infinite numbers of possible intermediate conformations between the two extreme
conformations are referred as skew conformations.
The
stabilities of various conformations of ethane are
Staggered
> Skew > Eclipsed
The
potential energy difference between the staggered and eclipsed conformation of
ethane is around 12.5 KJmol-1. The various conformations can be represented by
new man projection formula.
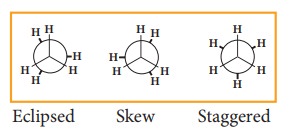
projection
formula for Ethane
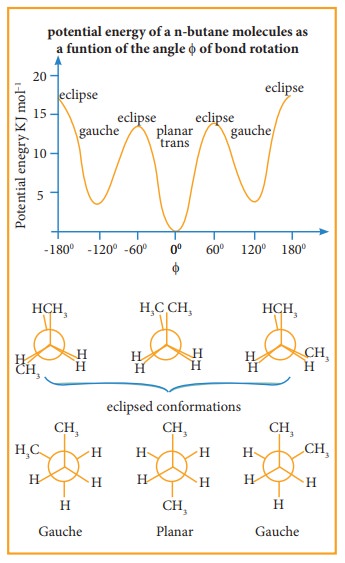
Conformations of n-Butane:
n-Butane
may be considered as a derivative of ethane, as one hydrogen on each carbon is
replaced by a methyl group
Eclipsed conformation:
In
this conformation, the distance between the two methyl group is minimum. So
there is maximum repulsion between them and it is the least stable conformer.
Anti or staggered form
In
this conformation, the distance between the two methyl groups is maximum and so
there is minimum repulsion between them. And it is the most stable conformer.
The
following potentially energy diagram shows the relative stabilities of various
conformers of n-butane.
Chemical properties:
Alkanes
are quite unreactive towards most reagents. However under favorable conditions,
alkanes undergo the following type of reaction.
Paraffin is the older name for the alkane group family of
compounds. This name comes from the Latin which means ‘little activity’
1) Combustion:
A
combustion reaction is a chemical reaction between a substances and oxygen with
evolution of heat and light (usually as a flame). In the presence of sufficient
oxygen, alkanes undergoes combustion when ignited and produces carbondioxide
and water.
The
combustion reaction is expressed as follows
for
example:
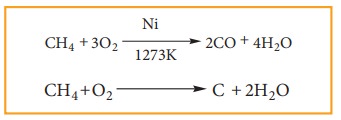
CH4 +2O2 → CO2 + 2H2O
ΔH°=-890.4kJ
When
alkanes burn in insufficient supply of oxygen, they form carbonmonoxide and
carbon black.
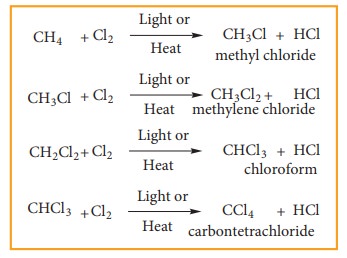
2) Halogenation:
Ahalogenation
reaction is the chemical reaction between an alkane and halogen in which one or
more hydrogen atoms are substituted by the halogens.
Chlorination
and Bromination are two widely used halogenation reactions. Fluorination is too
quick and iodination is too slow. Methane reacts with chlorine in the presence
of light or when heated as follows.
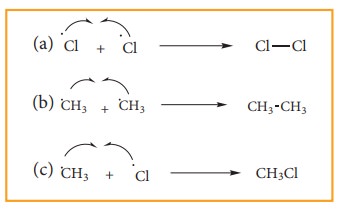
Mechanism:
The
reaction proceeds through the free radical chain mechanism. This mechanism is
characterized by three steps initiation, propagation and termination.
i)
CHAIN INITITATION: The chain is initiated by UV light leading to homolytic
fission of chlorine molecules into free radicals (chlorine atoms).
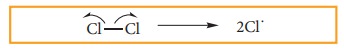
Here
we choose Cl-Cl bond for fission because C-C & C-H bonds are stronger than
Cl-Cl.
ii)
PROPAGATION: It proceeds as follows,
a)
Chlorine free radial attacks the methane molecule and breaks the C-H bond
resulting in the generation of methyl free radical
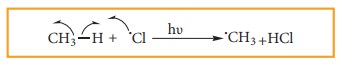
b)
The methyl free radical thus obtained attacks the second molecule of chlorine
to give chloromethane (CH3Cl) and a chlorine free radical as
follows.
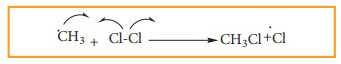
c)
This chlorine free radical then cycles back to step (a) and both step (a) and
(b) are repeated many times and thus chain of reaction is set up.
iii) Chain termination:
After
sometimes, the reactions stops due to consumption of reactant and the chain is
terminated by the combination of free radicals.
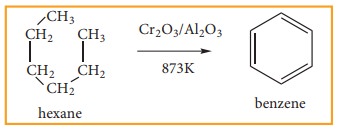
3) Aromatisation
Alkanes
with six to ten carbon atoms are converted into homologous of benzene at high
temperature and in the presence of catalyst. This process is known as
aromatization. It occurs by simultaneous cyclisation followed by
dehydrogenation of alkanes.
n-Hexane
passed over Cr2O3 supported on alumina at 873 K gives
benzene.
4) Reaction With Steam:
Methane
reacts with steam at 1273K in the presence of Nickel and decomposes to form
carbon monoxide and hydrogen gas.
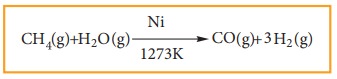
Production
of H2 gas from methane is known as steam reforming process and it is
a well-established industrial process for the production of H2 gas
from hydrocarbons.
5) Pyrolysis
Pyrolysis
is defined as the thermal decomposition of organic compound into smaller
fragments in the absence of air through the application of heat. ‘Pyro’ means
‘fire’ and ‘lysis’ means ‘separating’. Pyrolysis of alkanes also named as
cracking.
In
the absence of air, when alkane vapours are passed through red-hot metal it
breaks down into simpler hydrocarbons.
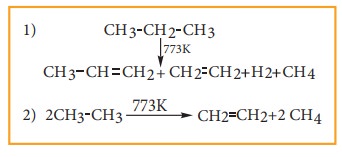
The
products depends upon the nature of alkane, temperature, pressure and presence
or absence of catalyst. The ease of cracking in alkanes increases with increase
in molecular weight and branching in alkanes. Cracking plays an important role
in petroleum industry.
6) Isomerisation:
Isomerisation
is a chemical process by which a compound is transformed into any its isomeric
forms. Normal alkanes can be converted into branched alkanes in the presence of
AlCl3 and HCl at 298 k.
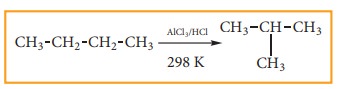
This
process is of great industrial importance. The quality of gasoline is improved
by isomerising its components.
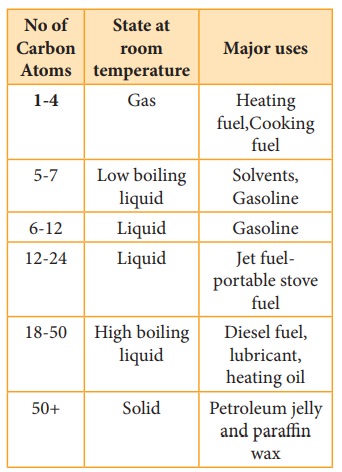
Uses
The
exothermic nature of alkane combustion reaction explains the extensive use of
alkanes as fuels. Methane present in natural gas is used in home heating.
Mixture of propane and butane are known as LPG gas which is used for domestic
cooking purpose. GASOLINE is a complex mixture of many hydrocarbons used as a
fuel for internal- combustion engines.
Carbon
black is used in the manufacture of ink, printer ink and black pigments. It is
also used as fillers.
Related Topics