Chapter: 11th 12th std standard Class Physics sciense Higher secondary school College Notes
Molecular theory of surface tension
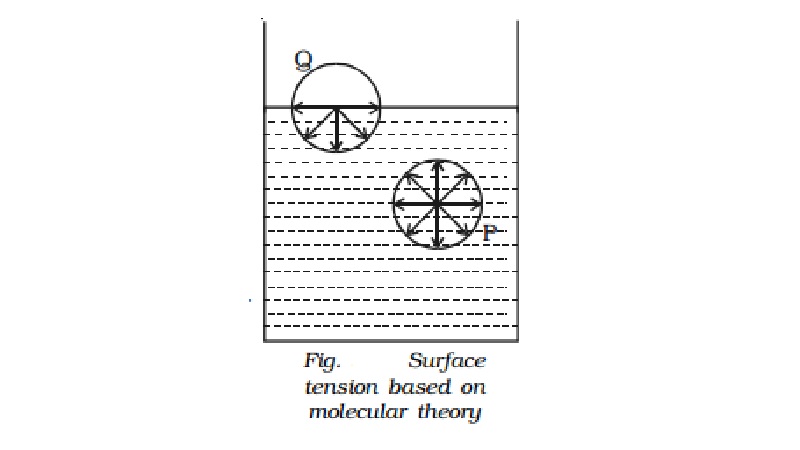
Molecular theory of surface tension
Consider two molecules P and Q as shown in Fig.. Taking them as
centres and molecular range as radius, a sphere of influence is drawn around
them.
The molecule P is attracted in all directions equally by
neighbouring molecules. Therefore net force acting on P is zero. The molecule Q
is on the free surface of the liquid. It experiences a net downward force
because the number of molecules in the lower half of the sphere is more and the
upper half is completely outside the surface of the liquid. Therefore all the
molecules lying on the surface of a liquid experience only a net downward
force.
If a molecule from the interior is to be brought to the surface of
the liquid, work must be done against this downward force. This work done on
the molecule is stored as potential energy. For
equilibrium, a system must possess
minimum potential energy. So, the free surface will have minimum potential energy. The free surface of a liquid tends
to assume minimum surface area by contracting and remains in a state of tension
like a stretched elastic membrane.
Surface tension of a liquid
Surface tension is the property of the free surface of a liquid at
rest to behave like a stretched membrane in order to acquire minimum surface
area.
Imagine a line AB in the free surface of a liquid at rest (Fig.).
The force of surface tension is measured as the force acting per unit length on
either side of this imaginary line AB. The force is perpendicular to the line
and tangential to the liquid surface. If F is the force acting on the length l
of the line AB, then surface tension is given by T=F/l.
Surface tension is defined as the force per unit length acting
perpendicular on an imaginary line drawn on the liquid surface, tending to pull
the surface apart along the line. Its unit is N m-1 and dimensional
formula
is MT-2.
Related Topics