Chapter: Modern Analytical Chemistry: Equilibrium Chemistry
Ladder Diagrams for Acid–Base Equilibria
Ladder Diagrams for Acid–Base Equilibria
To see how a ladder
diagram is constructed, we will use the acid–base equilibrium between HF and F–

Finally, replacing the negative log terms with p-functions and rearranging leaves
us with
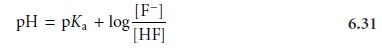
Examining equation 6.31 tells us a great deal about the
relationship between pH and the
relative amounts of F– and HF at equilibrium. If the concentrations of F– and HF are equal,
then equation 6.31 reduces to
pH=
pKa,HF = –log(Ka,HF) = –log(6.8 x 10–4) = 3.17
For concentrations of F– greater than that of HF, the
log term in equation 6.31
is positive and
pH>
pKa,HF or pH
> 3.17
This is a reasonable result since we expect the concentration of hydrofluoric acid’s conjugate base, F–, to increase as the pH increases. Similar
reasoning shows that the
concentration of HF exceeds that
of F–
when
pH < pKa,HF
or pH < 3.17
Now
we
are
ready
to
construct the ladder diagram for HF (Figure 6.4).
The
ladder
diagram
consists
of
a vertical
scale of pH values oriented so that smaller
(more acidic) pH levels are at the bottom and larger (more basic) pH levels
are at the top. A horizontal line is drawn
at a pH equal to pKa,HF. This line, or step,
separates the solution
into regions where
each of the two conjugate forms of HF predominate.
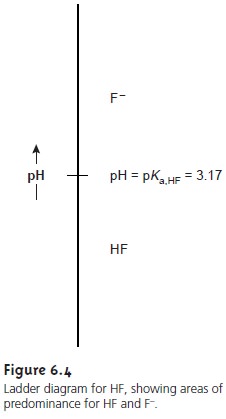
By
referring to the ladder diagram, we see that at a pH of 2.5 hydrofluoric acid will exist
predominately as HF. If we add sufficient base to the solution
such that the pH increases to 4.5, the
predominate form be- comes F–.
Figure 6.5 shows a second ladder diagram containing information
about HF/F– and NH4 +/NH3 . From
this ladder diagram
we see that if the pH is less
than 3.17, the predominate species
are HF and
NH4+. For
pH’s between 3.17 and 9.24 the predominate species are F– and NH4+, whereas above a pH of 9.24
the predominate species are F– and NH3.
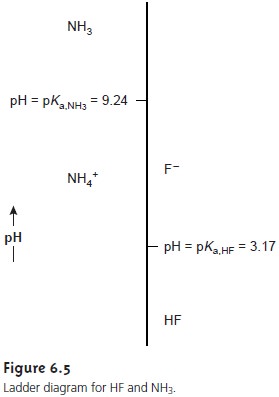
Ladder diagrams are
particularly useful for
evaluating the reactivity of acids and bases.
An acid and a base cannot coexist
if their respective areas of
predominance do not overlap. If we mix together solutions of NH3 and HF,
the reaction
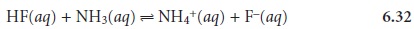
occurs because
the predominance areas
for HF and NH3 do not overlap.
Be- fore continuing, let us show that this conclusion is reasonable by calculating
the equilibrium constant
for reaction 6.32.
To do so we need the following three reactions and their
equilibrium constants.

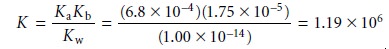
Since the equilibrium constant is significantly greater than 1, the reaction’s equilib- rium position lies far to the right.
This conclusion is general and applies to all lad- der
diagrams. The following example shows how we can use the ladder diagram
in Figure 6.5 to evaluate the composition of any solution
prepared by mixing together
solutions of HF and NH3.

If the areas
of predominance for an acid and a base overlap
each other, then practically no reaction occurs.
For example, if we mix together solutions
of NaF and NH4Cl, we expect that there will be no significant change in the moles of F– and NH4+. Furthermore, the pH of the mixture
must be between
3.17 and 9.24.
Because F– and NH4+ can coexist over a range of pHs we cannot be more specific in estimat- ing the solution’s pH.
The
ladder
diagram
for
HF/F– also can be used to evaluate the effect of
pH on other
equilibria that include
either HF or F–. For
example, the solubility of CaF2
CaF2(s) < = = = > tCa2+(aq)+ 2F–(aq)
is affected by pH because
F– is a weak base.
Using Le Châtelier’s principle, if F– is
converted to HF, the solubility of CaF2 will
increase. To minimize the solubility of CaF2 we want to control the solution’s pH so that F– is the predominate species. From the ladder diagram
we see that maintaining a pH of more than 3.17 ensures that solubility losses are minimal.
Related Topics