Chapter: 11th 12th std standard Class Physics sciense Higher secondary school College Notes
Isothermal process and Workdone in an isothermal expansion
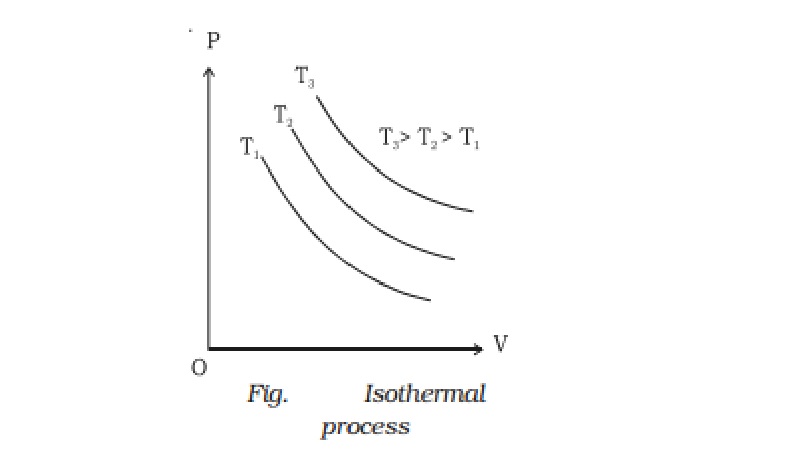
Isothermal process
When a gas undergoes expansion or
compression at constant temperature, the process is called isothermal process.
Let us consider a gas in a
cylinder provided with a frictionless piston. The cylinder and the piston are
made up of conducting material. If the piston is pushed down slowly, the heat
energy produced will be quickly transmitted to the surroundings. Hence, the
temperature remains constant but the pressure of the gas increases and its
volume decreases.
The equation for an isothermal
process is PV = constant.
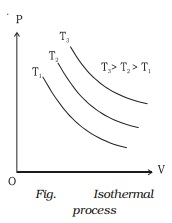
If a graph is drawn between P and V, keeping temperature constant, we get a curve called an
isothermal curve. Isotherms for three different temperatures T1, T2 and T3 are shown in the Fig.. The
curve moves away from the origin at higher temperatures.
During an isothermal change, the
specific heat capacity of the gas is infinite.
( i.e) C = ∆Q/m ∆T = infinity
(e.g) Melting of ice at its
melting point and vapourisation of water at its boiling point.
Workdone in an isothermal
expansion
Consider one mole of an ideal gas
enclosed in a cylinder with perfectly conducting walls and fitted with a
perfectly frictionless and conducting piston. Let P1, V1 and T be the initial pressure, volume and temperature of the gas. Let
the gas expand to a volume V2
when pressure reduces to P2,
at constant temperature T. At any
instant during expansion let the pressure of the gas be P. If A is the area of cross section of the piston, then force F = P ? A.
Let us assume that the pressure
of the gas remains constant during an infinitesimally small outward
displacement dx of the piston. Work
done
dW = Fdx = PAdx = PdV
Total work done by the gas in
expansion from initial volume V1
to final volume V2 is
W = ∫v1v2
PdV
We know, PV = RT, P =
RT/V
W = ∫v1v2 RT/V dV = RT ∫v1v2 1/V dV
W = RT [logeV]v1v2
W = RT [logeV2
- logeV1]
= RTloge (V2/V1)
This is the equation
for the workdone during an isothermal process.
Related Topics