Chapter: Modern Analytical Chemistry: Equilibrium Chemistry
A More Complex Problem: The Common Ion Effect
A More Complex Problem: The Common Ion Effect
Calculating the solubility of Pb(IO3)2 in distilled water
is a straightforward prob-
lem since the dissolution of the solid
is the only
source of Pb2+ or IO3–. How is the solubility of Pb(IO3)2 affected if we add Pb(IO3)2 to a solution
of 0.10 M Pb(NO3)2? Before
we set up and solve
the problem algebraically, think about the chemistry occurring in this system, and decide whether
the solubility of Pb(IO3)2
will
increase, decrease, or remain the same. This is a good habit to develop. Knowing what answers are reasonable will help you spot errors
in your calcula- tions and give you
more confidence that
your solution to a problem
is correct.
We begin by setting up a table
to help us keep track
of the concentrations of P b2+ and IO3– in this system.
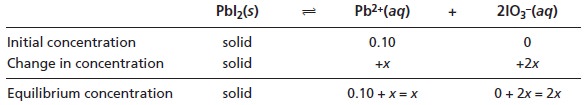
Substituting the equilibrium concentrations into the solubility product
expression (equation 6.33)
(0.10 + x)(2x)2 = 2.5 x 10–13
and multiplying out the terms on the left leaves us with
4x3 +
0.40x2 = 2.5
x 10–13
This is a more difficult equation to solve
than that for the solubility of Pb(IO3)2 in dis-
tilled water, and its solution
is not immediately obvious. A rigorous solution
to equa- tion 6.34 can be found using
available computer software
packages and spreadsheets.
How
might
we solve equation
6.34 if we do not have access to a computer?
One possibility is that we can apply our understanding of chemistry
to simplify the algebra. From Le Châtelier’s principle, we expect that the large initial concentration of Pb2+ will
significantly decrease
the solubility of Pb(IO3)2. In this case we can reasonably expect the
equilibrium concentration of Pb2+ to be very close to its initial
concentration; thus, the
following approximation for the equilibrium concentration of Pb2+ seems reasonable
[Pb2+] = 0.10 + x = 0.10 M
Substituting into equation 6.34

Before accepting this answer, we check to see if our approximation was reasonable. In this
case the approximation 0.10 + x = 0.10 seems
reasonable since the
difference between the two values is negligible. The equilibrium concentrations of Pb2+ and IO3–, therefore, are
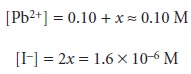
The solubility of Pb(IO3)2 is equal to the additional concentration of Pb2+ in solu- tion, or 7.9 x 10–7 mol/L. As expected, the solubility of Pb(IO3)2 decreases in the presence of a solution that already contains one of its
ions. This is known as the
common ion effect.
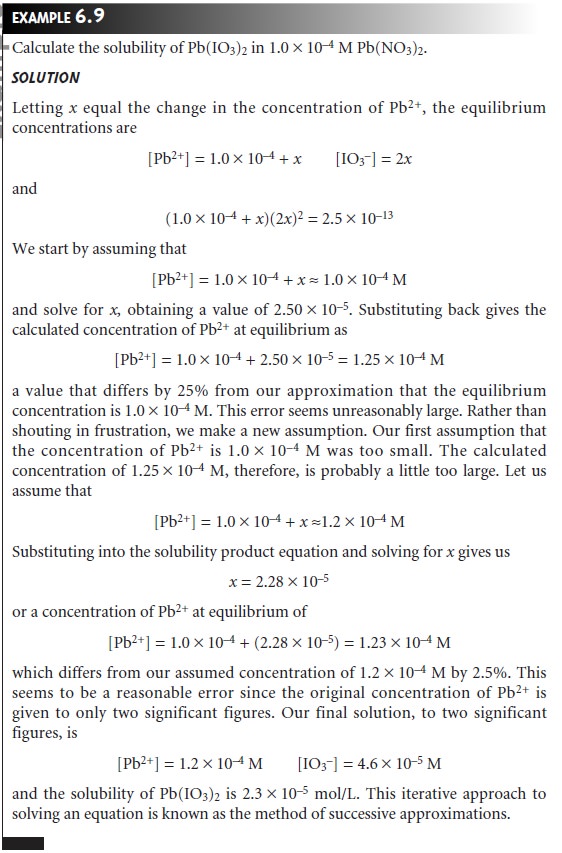
As outlined in the following example, the process
of making and evaluating ap- proximations can be extended if the
first approximation leads to an unacceptably
large error.
Related Topics