Chapter: Psychology: Psychopathology
The Roots of Schizophrenia
The Roots of Schizophrenia
What
leads to these symptoms? Like the other causes of disorders we have discussed,
the causes of schizophrenia are complex, involving genetic, prenatal, neural,
social, and psychological factors.
GENETIC AND PRENATA FACTORS
It
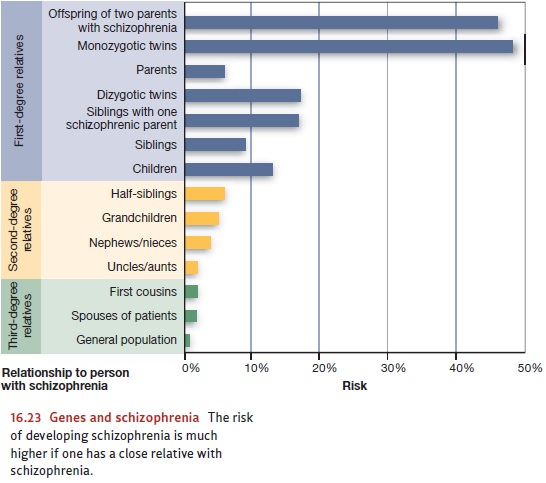
has long been known that schizophrenia runs in families (Figure 16.23). For
example, if a person has a sibling with schizophrenia, the likelihood that he
has (or will get) the disease himself is four times greater than the likelihood
of schizophre-nia in the general population—8%, compared with 1 or 2% in the
broad population (Andreasen & Black, 1996; D. Rosenthal, 1970). But this
fact, by itself, does not prove a genetic contribution. (After all, the
increased risk among siblings might reflect a factor in the home or family
environment.) Better evidence comes from concordance rates between twins, which
are between 41 and 65% if twins are identical, and between 0 and 28% if they
are fraternal (Cardno & Gottesman, 2000).
Separate evidence comes from adoption studies. Consider a child who is born to a mother with schizophrenia and placed in a foster home (with foster parents who are not schizophrenic) within a week or so after birth. The odds are about 8% that this child will develop schizophrenia, the same percentage as for children who remain with a biological parent who has the disease (Kendler & Gruenberg, 1984; Kety, 1988; Tsuang, Gilbertson, & Faraone, 1991).
All
of these findings suggest that there is some genetic contribution to the
develop-ment of schizophrenia (Sullivan, Kendler, & Neale, 2003), and, in
fact, scientists may be closing in on the specific genes that are responsible.
For example, two separate stud-ies have found that people with two specific DNA
deletions are substantially more likely than people without these deletions to
develop schizophrenia (International Schizophrenia Consortium, 2008; Stefansson
et al., 2008).
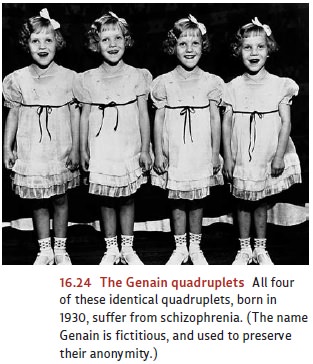
However,
if genes were the whole story for schizophrenia, the concordance rate would be
100% for identical twins, and of course it is not. In addition, if genes were
all that mattered, we would expect that genetically identical individuals who
develop schizophrenia will have identical symptom profiles. This is not the
case. For example, the genetically identical Genain quadruplets (see Figure
16.24) all have schizophrenia, but two of the sisters have a more serious
symptom profile than the others. These observations lead us to ask what other
(nongenetic) factors play a role. In recent years, attention has focused on
factors associated with birth—both prenatal factors and factors during
delivery.
One
line of evidence suggests that the mother’s exposure to an infectious agent dur-ing
pregnancy may increase the likelihood that her child will develop
schizophrenia. The influenza virus has attracted special attention based on the
finding that when mothers are in the second trimester of pregnancy during an
influenza epidemic, their children are more likely to develop schizophrenia
(Brown, Cohen, Harkavy-Friedman, & Babulas, 2001; Mednick, Huttunen, &
Macho’n, 1994; Sham et al., 1992). These find-ings are supported by
epidemiological studies that show that children who develop schizophrenia are
disproportionately likely to have been born during the winter (January to March
in the Northern Hemisphere, July to September in the Southern
Hemisphere), the season during which people stay inside more and thus share more viral infections. In geographic areas where there are no seasons—that is, in areas near the equator—there is no link between schizophrenia and birth month (Battle, Martin, Dorfman, & Miller, 1999; McGrath, Welham, & Pemberton, 1995; Parker, Mehendran, Koh, & Machin, 2000).
Infections,
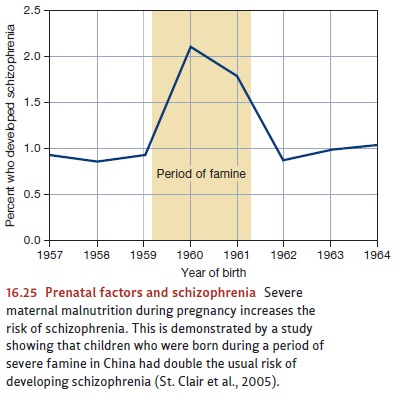
however, are likely not the whole story. Another line of evidence suggests that
maternal malnutrition during pregnancy also increases the risk that the child
will later develop schizophrenia (Figure 16.25). Thus, children born just after
a major crop failure in China in the early 1960s were twice as likely as
Chinese children born earlier or later to develop schizophrenia (St. Clair et al.,
2005).
Evidence
further suggests that a diverse set of birth complications is associated with
schizophrenia, and what these complications have in common seems to be a period
of diminished oxygen supply to the newborn. This oxygen deprivation by itself
is not enough to produce the disease, but it may interfere with the newborn’s
brain develop-ment in a way that increases the likelihood that a genetic
predisposition will eventually be expressed as schizophrenia (T. D. Cannon et
al., 2000; Zorilla & Cannon, 1995).
We
still need to ask, though, what these various factors do to produce the illness
we call schizophrenia. What are the effects of the infection, or the oxygen
deprivation, or the genetic pattern associated with this illness? According to
many investigators, the answer lies in the fact that schizophrenia is, at its
heart, a neurodevelopmentaldisorder (Sawa
& Snyder, 2002; Waddington, Torrey, Crow, & Hirsch, 1991). In
otherwords, the various factors we have mentioned cause the child’s brain (in both
its structure and its chemistry) not to develop as it should from a fairly
early age. By this logic, though schizophrenia may not be diagnosed until
adolescence, it reflects developmental problems that occurred years earlier.
Consistent
with this notion, evidence suggests that many individuals who are even-tually
diagnosed with this illness are, in fact, unusual in early childhood. For
example, close examination of home movies of children who, years later, were
diagnosed with schizophrenia reveals that the “preschizophrenic children”
showed less positive emotion in their facial expressions and more negative
facial emotion, compared with siblings who did not later develop schizophrenia
(Walker, Grimes, Davis, & Smith, 1993; Walker, Kestler, Bollini, & Hochman,
2004; Walker, Savoie, & Davis, 1994). The preschizo-phrenic children also
showed unusual motor patterns, including odd hand movements. In some cases,
these differences were visible at a very early age—as young as 2 years
old—which strongly suggests that the disease starts influencing the person in
early childhood, even if the full disruption it causes is not detected for
years (Figure 16.26).

BRAIN BASES
Clearly,
genetic factors and environmental factors before and during birth contribute to
schizophrenia and seem to throw brain develop-ment somehow off-course. But
off-course how? Can we pinpoint the
biological changes that these various factors produce, which then lead to the
illness?
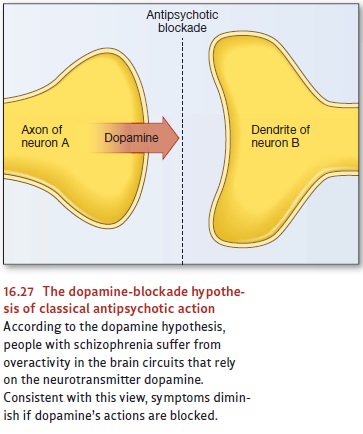
According
to the dopamine hypothesis,
schizophrenia is associated with an abnormally high level of activity in the
brain circuits sensitive to the neurotransmitter dopamine. The strongest line
of evidence for this hypothesis comes from the effects of a number of
medications known as classical
antipsychotics, medications that include the drugs Thorazineand Haldol.
These drugs block receptors for dopamine (Figure 16.27), and, as the dopamine
hypothesis predicts, they relieve many of the symp-toms associated with
schizophrenia. In addition, some antipsychotics are more effective than others
in blocking dopamine receptors, and the stronger the blockade, the more
therapeutic the drug.
Other
evidence comes from people who do not have schizophrenia but who have taken
overdoses of amphetamines. Amphetamines are stimulants whose effects include
the enhancement of dopamine activity and, when taken in large enough doses,
produce a temporary psychosis similar to schizophrenia. As the dopamine
hypothesis would predict, medications that block dopamine activity at the
synapse also reduce the psychotic symptoms that follow amphetamine abuse.
The
dopamine hypothesis has much to recommend it, but in recent years investigators
have realized that it is incomplete (Carlsson et al., 1995). One clue is that
many of the newer antipsychotic medications—which are at least as effective as
older antipsychotics but generally have fewer side effects—do not appear to be
strong dopamine antagonists (Burris et al., 2002). Researchers now believe that
people with schizophrenia may suffer both from excessive dopamine stimulation
in some brain circuits (Laruelle, Kegeles, & Abi-Darham, 2003) and from
insufficient dopamine stimulation elsewhere (e.g., in the prefontal cortex;
Koh, Bergson, Undie, Goldman-Rakic, & Lidow, 2003).
Other
neurotransmitter systems also seem to be implicated in schizophrenia. For
example, people with schizophrenia may have a dysfunction in glutamate
transmis-sion in their brains, either because they have insufficient glutamate
or because they are relatively insensitive to it. Several pieces of evidence
point in this direction, includ-ing the fact that the illicit drug
phencyclidine (more commonly known as PCP or angel dust) blocks glutamate
receptors and induces symptoms similar to those seen in schizophrenia (Gorelick
& Balster, 1995). In addition, drugs that increase glutamate activity
alleviate both positive and negative symptoms of schizophrenia (Goff &
Coyle, 2001).
We
probably should not think of the dopamine and glutamate proposals as
competi-tors; both might capture part of the truth. Indeed, this reflects one
of the messages emerging from recent research on schizophrenia: Multiple
neurotransmitters seem to be involved, affecting multiple brain areas, under
the control of multiple genes (cf. Javitt & Coyle, 2004; Sawa & Snyder,
2002).
In
addition to the neurochemical disruptions in schizophrenia, research indicates
that patients with this disorder also suffer from structural abnormalities in
their brains. MRI scans show that a certain proportion of people with
schizophrenia—males, especially—have an enlargement of the ventricles, the
fluid-filled cavities in the brain. Simply put, the ventricles become enlarged
because there is not enough brain to fill the
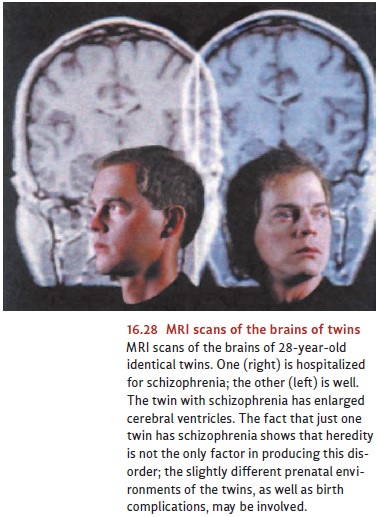
skull
(Figure 16.28). This finding indicates that in many cases of schizophrenia,
there is either a dramatic loss of brain tissue or a defi-ciency that existed
from the start (Andreasen et al., 1986; Chua & McKenna, 1995; Lawrie &
Abukmeil, 1998; Nopoulos, Flaum, & Andreasen, 1997).
Abnormalities
associated with schizophrenia have also been reported in other areas of the
brain (Heckers, 1997; L. K. Jacobsen et al., 1997), but the most persuasive
findings involve the frontal and temporal lobes (Black & Andreasen, 1994;
Martin & Albers, 1995). Studies of brain structure have documented a loss
of gray matter in prefrontal regions that support working memory, and the
degree of tis-sue loss seems to be correlated with symptom severity (Cannon et
al., 2002). When these areas are examined during autopsy, individuals with
schizophrenia also show various irregularities, including missing or abnormally
sized neurons. These neuronal defects—not surprisingly—affect overall brain
function: neuroimag-ing studies of patients with schizophrenia indicate
atypical functioning in the areas where neuronal defects are common (Barch et
al., 2001; Tan et al., 2006).
SOCIAL AND PSYCHOLOGICAL RISK FACTORS
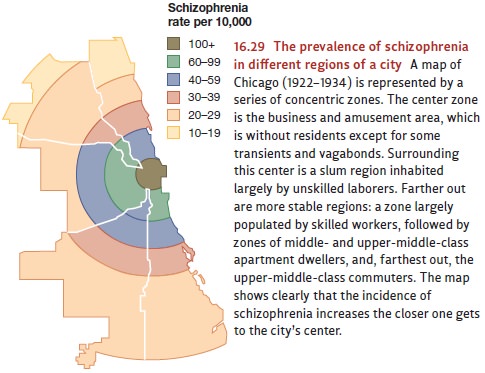
Almost
a century ago, epidemiological studies revealed a link between schizophrenia
and socioeconomic status, or SES (Faris & Dunham, 1939). In fact, one study
suggested that low-SES individuals are nine times more likely to develop
schizophrenia than are high-SES individuals (Hollingshead & Redlich, 1958).
The same point can be made geographically, since the prevalence of
schizophrenia is highest in the poorest and most dilapidated areas of a city
and diminishes as one moves toward higher-income regions (Figure 16.29; M. L.
Kohn, 1968).
What
produces this relationship? Part of the answer is, sadly, daily stress—poverty,
inferior status, and low occupational rank are all stressful, and so can help
trigger schiz-ophrenia in someone who is (for biological reasons) already
vulnerable (Goldberg & Morrison, 1963). But there is another reason why
schizophrenia is associated with poverty: Someone who suffers from
schizophrenia is less likely to do well in school and less likely to get or
hold a good job. As a result, people with schizophrenia suffer from downward drift. Their disease pro-duces
problems that, in turn, put them into a lower social class (Dohrenwend et al.,
1992; Jones et al., 1993). Notice, then, that cause and effect run in both
directions here: Poverty is a risk factor for schizophrenia, making the disease
more likely, but schizophre-nia is itself a risk factor that makes poverty more
likely.
What about someone’s immediate environment—for example, her family? Some investigators have looked to the per-sonality of a person’s parents as a potential source of schizophrenia. Others have focused on communication patterns in the family (Bateson, 1959, 1960). There is little evidence, however, in favor of either of these claims. In fam-ilies that include someone with schizophrenia disturbances may be common, but this is likely to be a consequence of the disease rather than its cause. After all, having a fam-ily member who suffers from schizophrenia can be tragic for the family. Parents often blame themselves for their child’s illness and are likely to become frustrated and despondent in their attempts to reach their child (Mishler & Waxler, 1968; Torrey, 1983).
Children
with schizophrenia may have difficult parents for another reason. Given the
link between schizophrenia and genetics, a child with schizophrenia is likely
to have at least one parent with the same pathological genes as the child’s.
Thus, the parents may have a muted (or perhaps just an undiagnosed) version of
the disease, contribut-ing to the family’s problems (Holtzman et al., 1988;
Reveley, Reveley, & Clifford, 1982; Tsuang et al., 1991).
In
short, there is no reason to believe that poor familial relations cause the
disorder. But the family context surely matters in other ways, including how
well a person with schizophrenia copes with the disorder. This is reflected in
the fact that patients, once treated and released from a hospital, are
rehospitalized more often if their parents are hostile and critical toward them
(Hooley, 2004). Such negative reactions from family members are likely to
impede the patient’s adjustment to his disorder and may create such distress
that another hospital stay becomes necessary.
Related Topics