Chapter: Biochemistry: Photosynthesis
The Relationship between Wavelength and Energy of Light
The
Relationship between Wavelength and Energy of Light
A well-known equation relates the wavelength and energy of light, a
point of crucial importance for our purposes. Max Planck established in the
early 20th century that the energy of light is directly proportional to its
frequency.
E = hν
whereE is energy, h is a constant (PlanckÕs constant), and
ν is the
frequency of the light. The wavelength of light is related to the frequency.
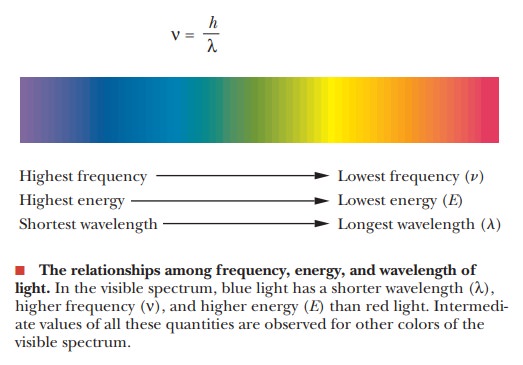
whereλ is
wavelength, ν is
frequency, and c is the velocity of
light. We can rewrite the expression for the energy of light in terms of
wavelength rather than frequency.
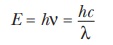
Light of shorter wavelength (higher frequency) is higher in energy
than light of longer wavelength (lower frequency).
Related Topics