Chapter: Plant Biochemistry: Photosynthesis is an electron transport process
The cytochrome-b6/f complex mediates electron transport between photosystem II and photosystem I
The cytochrome-b6/f complex mediates electron transport between photosystem II and photosystem I
Iron atoms in cytochromes and in iron-sulfur centers have a central function as redox carriers
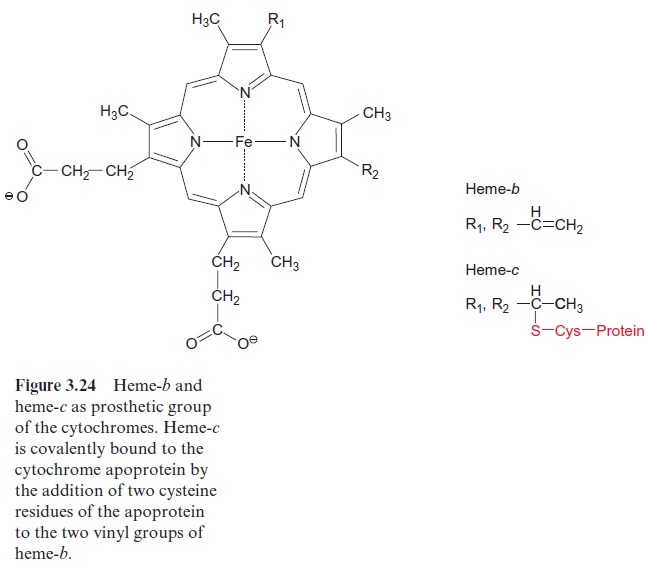
Cytochromes are divided into three main groups, the cytochromes-a, -b, and -c. These correspond to heme-a, -b, and -c. Heme-b may be regarded as the basic structure (Fig. 3.24). In heme-c the -SH-group of a cysteine is added to each of the two vinyl groups of heme-b. In this way heme-c is covalently bound by a sulfur bridge to the protein of the cytochrome. Such a mode of covalent binding has already been shown for phycocyanin in Figure 2.15, and there is actually a structural relationship between the correspond-ing apoproteins. In heme-a (not shown) an isoprenoid side chain consisting of three isoprene units is attached to one of the vinyl groups of heme-b. This side chain functions as a hydrophobic membrane anchor, similar to that found in quinones (Figs. 3.5 and 3.19). Heme-a is mentioned here only for the sake of completeness. It plays no role in photosynthesis, but it does have a function in the mitochondrial electron transport chain .
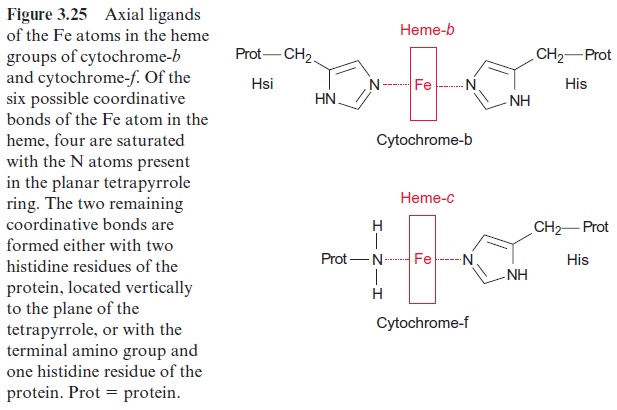
The iron atom in the heme can form up to six coordinative bonds. Four of these bonds are formed with the nitrogen atoms of the tetrapyrrole ring. This ring has a planar structure. The two remaining bonds of the Fe atom coordinate with two histidine residues, which are positioned vertically to the tetrapyrrole plane (Fig. 3.25). Cyt-f (f = foliar, in leaves) contains, like cyt-c, one heme-c and therefore belongs to the c-type cytochromes. In cyt-f one bond of the Fe atom coordinates with the terminal amino group of the protein and the other with a histidine residue.
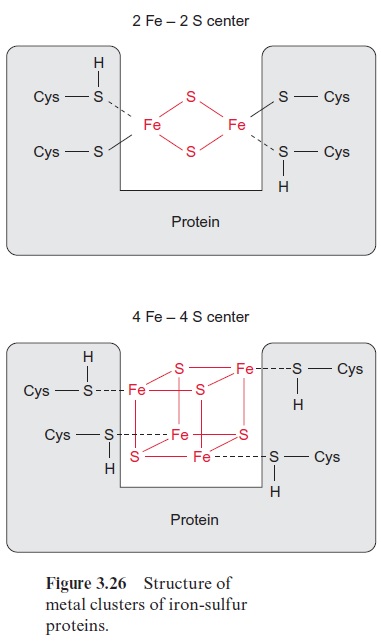
Iron-sulfur centers are of general importance as electron carriers in elec-tron transport chains and thus also in photosynthetic electron transport. Cysteine residues of proteins within iron-sulfur centers (Fig. 3.26) are coor-dinatively or covalently bound to Fe atoms. These iron atoms are linked to each other by S-bridges. Upon acidification of the proteins, the sulfur between the Fe atoms is released as H2S and for this reason it has been called labile sulfur. Iron-sulfur centers occur mainly as 2Fe-2S or 4Fe-4S centers. The Fe atoms in these centers are present in the oxidation states Fe++ and Fe+++ . Irrespective of the number of Fe atoms in a center, the oxidized and reduced state of the center differs only by a single charge. For this reason, iron-sulfur centers can take up and transfer only one electron. Various iron-sulfur centers have very different redox potentials, depending on the surrounding protein.
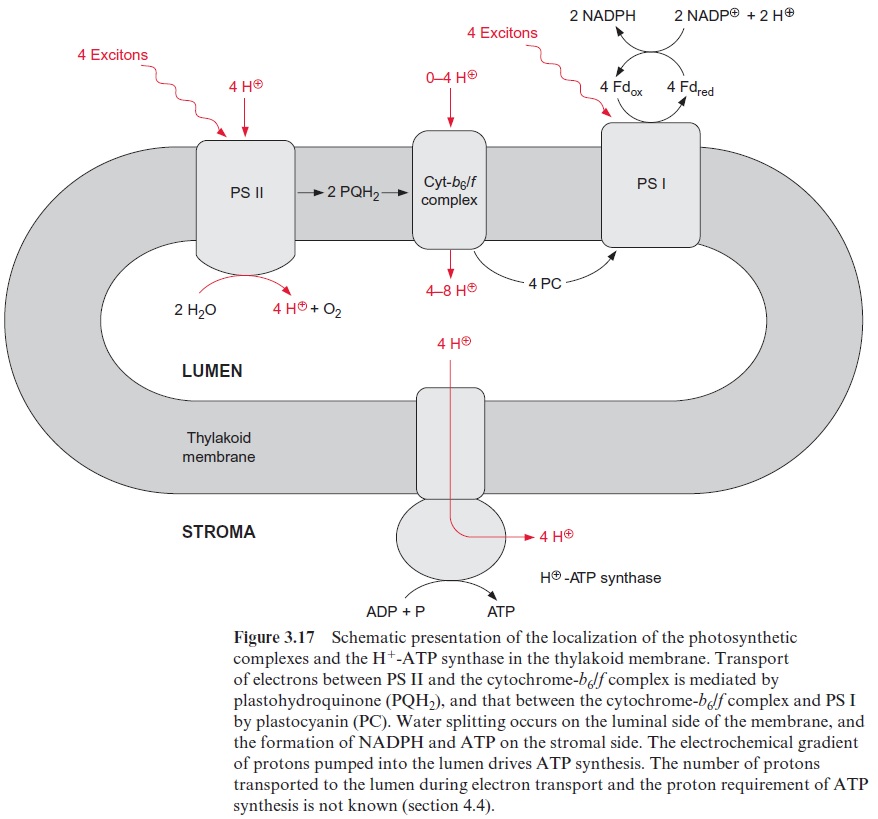
The electron transport by the cytochrome-b6/f complex is coupled to a proton transport
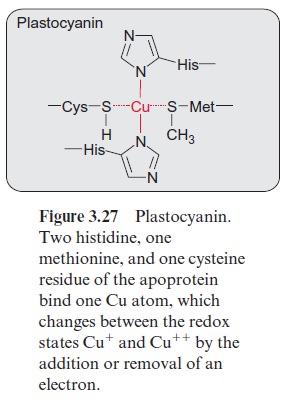
Plastohydroquinone (PQH2) formed by PS II diffuses through the lipid phase of the thylakoid membrane and transfers its electrons to the cytochrome-b6/f complex (Fig. 3.17). This complex then transfers the electrons to plastocy-anin, which is thus reduced. Therefore the cytochrome-b6/f complex has also been calledplastohydroquinone-plastocyanin oxidoreductase. Plastocyanin is a protein with a molecular mass of 10.5 kDa, containing a copper atom, which is coordinatively bound to one cysteine, one methionine, and two histidine residues of the protein (Fig. 3.27). This copper atom alternates between the oxidation states Cu+ and Cu++ and thus is able to take up and transfer one electron. Plastocyanin is soluble in water and is located in the thylakoid lumen.
Electron transport through the cyt-b6/f complex proceeds along a poten-tial difference gradient of about 0.4 V (Fig. 3.16). The energy liberated by the transfer of the electron down this redox gradient is conserved by trans-porting protons to the thylakoid lumen. The cyt-b6/f complex is a mem-brane protein consisting of at least eight subunits. The main components of this complex are four subunits: cyt-b6, cyt-f, an iron-sulfur protein called Rieske protein after its discoverer, and a subunit IV. Additionally, there are some smaller peptides and a chlorophyll and a carotenoid of unknown function. The Rieske protein has a 2Fe-2S center with the very positive redox potential of +0.3 V, untypical of such iron-sulfur centers.
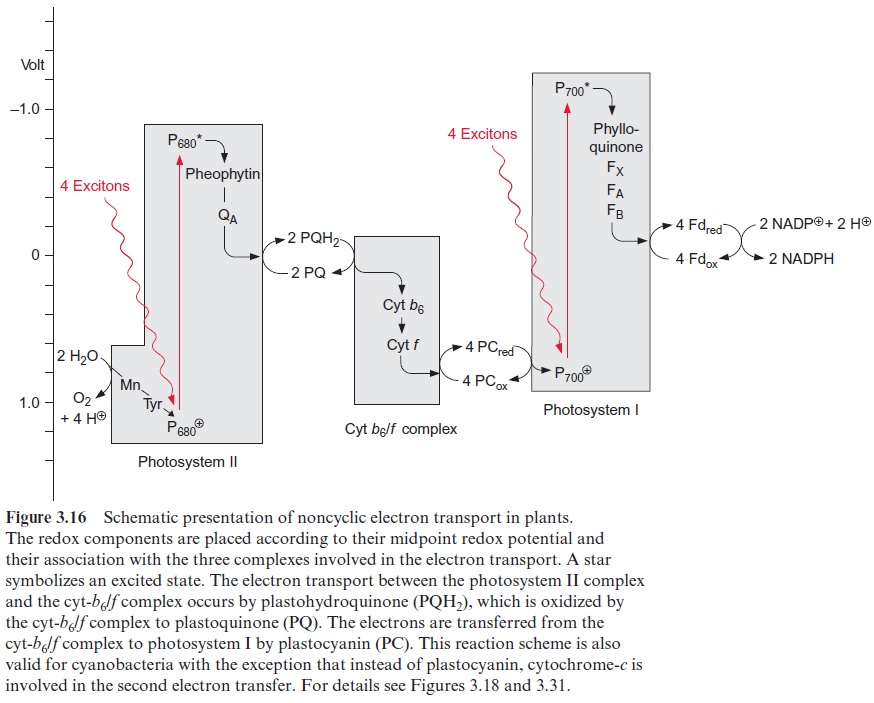
The cyt-b6/f complex has an asymmetric structure (Fig. 3.28). Cyt-b6 and subunit IV span the membrane. Cyt-b6 containing two heme-b molecules is almost vertically arranged to the membrane and forms a redox chain across the membrane. Cyt-b6 also contains a heme-c, of which the function has not been fully resolved and is therefore not shown in the figure. Cyt-b6 has two binding sites for PQH2/PQ, one in the region of the lumen and one in the region of the stroma. The function of these binding sites will be explained in Figures 3.29 and 3.30. The iron sulfur Rieske protein protrudes from the lumen into the membrane. Closely adjacent to it is cyt-f containing a binding site responsible for the reduction of plastocyanin. The Rieske protein and cyt-f are attached to the membrane by a membrane anchor.
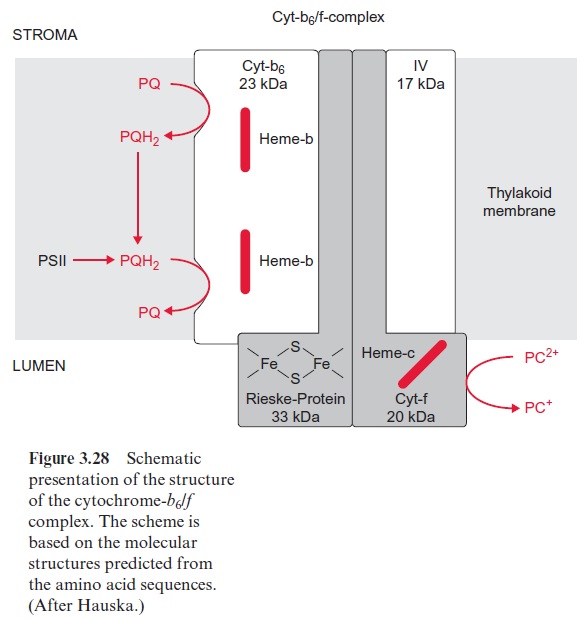
The cyt-b6/f complex resembles in its structure the cyt-b/c1 complex in bacteria and mitochondria . Table 3.3 summarizes the func-tion of these cyt-b6/f and cyt-b/c1complexes. All these complexes possess one iron-sulfur protein. The amino acid sequence of cyt-b in the cyt-b/c1 complex of bacteria and in mitochondria corresponds to the sum of the sequences of cyt-b6 and the subunit IV in the cyt-b6/f complex. Apparently during evolution the cyt-b gene was cleaved into two genes, for cyt-b6 and subunit IV. Whereas in plants the cyt-b6/f complex reduces plastocyanin, the cyt-b/c1 complex of bacteria and mitochondria reduces cyt-c. Cyt-c is a very small cytochrome molecule that is water-soluble and, like plasto-cyanin, transfers redox equivalents from the cyt-b6/f complex to the next complex along the aqueous phase. In cyanobacteria, which also possess a cyt-b6/f complex, the electrons are transferred from this complex to photo-system I via cyt-c instead of plastocyanin. The great similarity between the cyt-b6/f complex in plants and the cyt-b/c1 complexes in bacteria and mito-chondria suggests that these complexes have basically similar functions in photosynthesis and in mitochondrial oxidation: they are proton transloca-tors that are driven by a hydroquinone-plastocyanin (or -cyt-c) reductase.
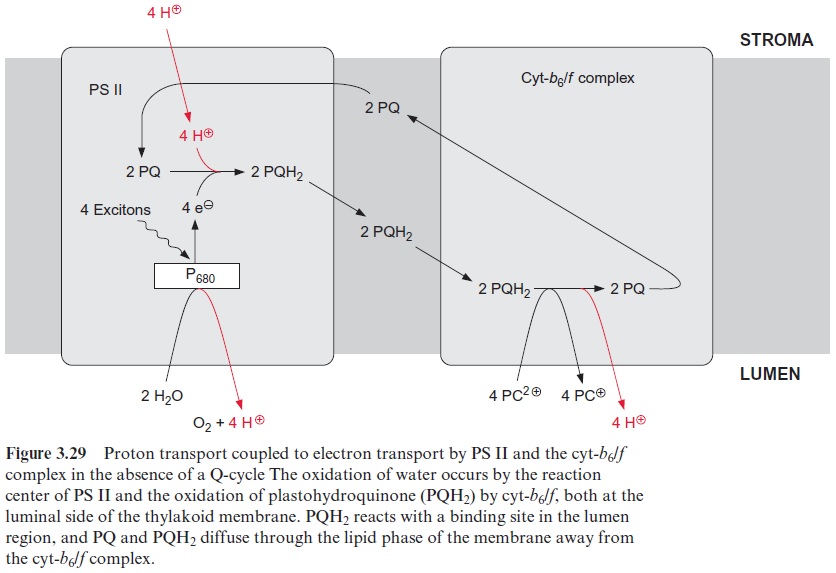
The interplay of PS II and the cyt-b6/f complex electron transport causes the transport of protons from the stroma space to the thylakoid lumen. The principle of this transport is explained in the schematic presentation of Figures 3.28 and 3.29. A crucial point is that the reduction and oxida-tion of the quinone occur at different sides of the thylakoid membrane. The required protons for the reduction of PQ (Qb) by the PS II complex are taken up from the stroma space. Subsequently PQH2 diffuses across the lipid phase of the membrane to the binding site in the lumenal region of the cyt-b6/f complex where it is oxidized by the Rieske protein and cyt-f to yield reduced plastocyanin. The protons of this reaction are released into the thylakoid lumen. According to this scheme, the capture of four excitons by the PS II complex transfers four protons from the stroma space to the lumen. In addition four protons produced during water splitting by PS II are released into the lumen as well.
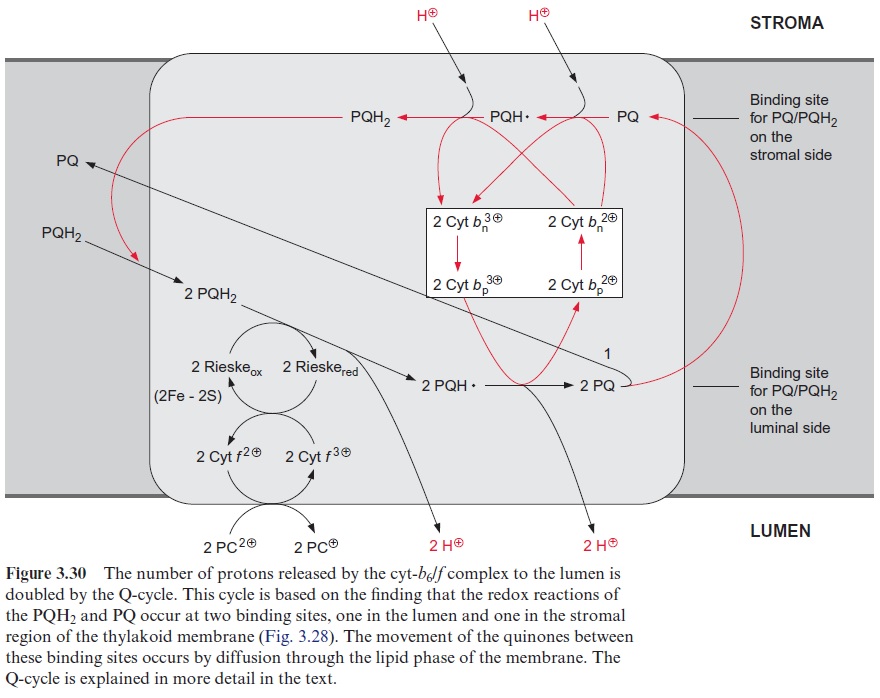
The number of protons pumped through the cyt-b6/f complex can be doubled by a Q-cycle
Studies with mitochondria indicated that during electron transport through the cyt-b/c1 complex, the number of protons transferred per transported electron is larger than four (Fig. 3.29). Peter Mitchell (Great Britain), who established the chemiosmotic hypothesis of energy conservation , also postulated a so-called Q-cycle, by which the number of trans-ported protons for each electron transferred through the cyt-b/c1 complex is doubled. It later became apparent that the Q-cycle also has a role in pho-tosynthetic electron transport
Figure 3.30 shows the principle of Q-cycle operation in the photosyn-thesis of chloroplasts. The cyt-b6/f complex contains two different bind-ing sites for conversion of quinones, one located at the stromal side and the other at the luminal side of the thylakoid membrane (Fig. 3.28). The plastohydroquinone (PQH2) formed in the PS II complex is oxidized by the Rieske iron-sulfur center at the binding site adjacent to the lumen. Due to its very positive redox potential, the Rieske protein tears off one electron from the plastohydroquinone. Because its redox potential is very negative, the remaining semiquinone is unstable and transfers its electron to the first heme-b of the cyt-b6 (bp) and from there to other heme-b (bn), thus rais-ing the redox potential of heme bn to about –0.1 V. In this way a total of four protons are transported to the thylakoid lumen per two molecules of plastohydroquinone oxidized. Of the two plastoquinone molecules (PQ) formed, only one molecule returns to the PS II complex. The other PQ dif-fuses away from the cyt-b6/f complex through the lipid phase of the mem-brane to the stromal binding site of the cyt-b6/f complex to be reduced via semiquinone to hydroquinone by the high reduction potential of heme-bn. This is accompanied by the uptake of two protons from the stromal space. The hydroquinone thus regenerated diffuses through the membrane back to the luminal binding site where it is oxidized in turn by the Rieske protein, and so on. In total, the number of transported protons is doubled by the Q-cycle (1/2+1/4+1/8+1/16…+ 1/n = 1). The fully operating Q-cycle transports four electrons through the cyt-b6/f complex which results in total to the transfer of eight protons from the stroma to the lumen. The func-tion of this Q-cycle in mitochondrial oxidation is now undisputed, while its function in photosynthetic electron transport is still a matter of contro-versy. The analogy of the cyt-b6/f complex to the cyt-b/c1 complex suggests that the Q-cycle also plays an important role in chloroplasts. So far, the operation of a Q-cycle in plants has been observed mainly under low light conditions. The Q-cycle is perhaps suppressed by a high proton gradient generated across the thylakoid membrane, for instance, by irradiation with high light intensity. In this way the flow of electrons through the Q-cycle could be adjusted to the energy demand of the plant cell
Related Topics