Chapter: Plant Biochemistry: Photosynthesis is an electron transport process
A reductant and an oxidant are formed during photosynthesis
A reductant and an oxidant are formed during photosynthesis
In the 1920s Otto Warburg (Berlin) postulated that the energy of light is transferred to CO2 and that the CO2, activated in this way, reacts with water to form a carbohydrate, accompanied by the release of oxygen. According to this hypothesis, the oxygen released by photosynthesis was derived from the CO2. In 1931 this hypothesis was opposed by Cornelis van Niel (USA) by postulating that during photosynthesis a reductant is formed, which then reacts with CO2. The so-called van Niel equation describes photosynthesis in the following way:
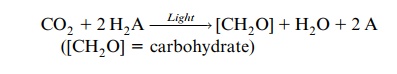
He proposed that a compound H2A is split by light energy into a reduc-ing compound (2H) and an oxidizing compound (A). For oxygenic photo-synthesis of cyanobacteria or plants, it can be rewritten as:
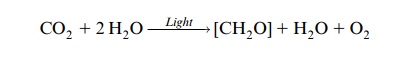
In this equation the oxygen released during photosynthesis is derived from water.
In 1937 Robert Hill (Cambridge, UK) proved that a reductant is actu-ally formed in the course of photosynthesis. He was the first to succeed in isolating chloroplasts with photosynthetic activity, which, however, had no intact envelope membranes and consisted only of thylakoid membranes. When these chloroplasts were illuminated in the presence of Fe3+ com-pounds (initially ferrioxalate, later ferricyanide ([Fe (CN)6]3- )), Robert Hill observed an evolution of oxygen accompanied by the reduction of the Fe3+ -compounds to the Fe2+ form.

This “Hill reaction” proved that the photochemical splitting of water can be separated from the reduction of the CO2. Therefore the complete reac-tion of photosynthetic CO2 assimilation can be divided into two reactions:
1. The so-called light reaction, in which water is split by photon energy to yield reductive power (NADPH) and chemical energy (ATP); and
2. the so-called dark reaction , in which CO2 is assimilated at the expense of the reductive power and of ATP.
In 1952 the Dutchman Louis Duysens made a very important observa-tion that helped explain the mechanism of photosynthesis. When illumi-nating isolated membranes of the purple bacterium Rhodospirillum rubrum with short light pulses, he found a decrease in light absorption at 890 nm, which was immediately reversed when the bacteria were darkened again. The same “bleaching” effect was found at 870 nm in the purple bacte-rium Rhodobacter sphaeroides. Later, Bessil Kok (USA) and Horst Witt (Germany) also found similar pigment bleaching at 700 nm and 680 nm in chloroplasts. This bleaching was attributed to the primary reaction of pho-tosynthesis, and the corresponding pigments of the reaction centers were named P870 (Rb. sphaeroides) and P680 and P700(chloroplasts). When an oxidant (e.g., [Fe(CN)6]3- ) was added, this bleaching effect could also be achieved in the dark. These results indicated that these absorption changes of the pigments were due to a redox reaction. This was the first indication that chlorophyll can be oxidized. Electron spin resonance measurements revealed that radicals are formed during this “bleaching.” “Bleaching” could also be observed at the very low temperature of 1 K. This showed that in the electron transfer leading to the formation of radicals, the reac-tion partners are located so close to each other that thermal oscillation of the reaction partners (normally the precondition for a chemical reaction) is not required for this redox reaction. Spectroscopic measurements indicated that the reaction partner of this primary redox reaction are two closely adjacent chlorophyll molecules arranged as a pair, called a “special pair.”
Related Topics