Chapter: Plant Biochemistry: Photosynthesis is an electron transport process
How does a reaction center function?
How does a reaction center function?
The analysis of the structure and extensive kinetic investigations allowed a detailed description of the function of the bacterial reaction center. The kinetic investigations included measurements by absorption and fluores-cence spectroscopy after light flashes in the range of less than 10 -13 s, as well as measurements of nuclear spin and electron spin resonance. Although the reaction center shows a symmetry with two almost identical branches of chromophores, electron transfer proceeds only along the right branch (the L side, Fig. 3.10). The chlorophyll monomer (BB) on the M side is in close contact with a carotenoid molecule, which abolishes a harmful triplet state of chlorophylls in the reaction center. The function of the pheophytin ( ФB) on the M side and of the non-heme iron is not yet fully understood.
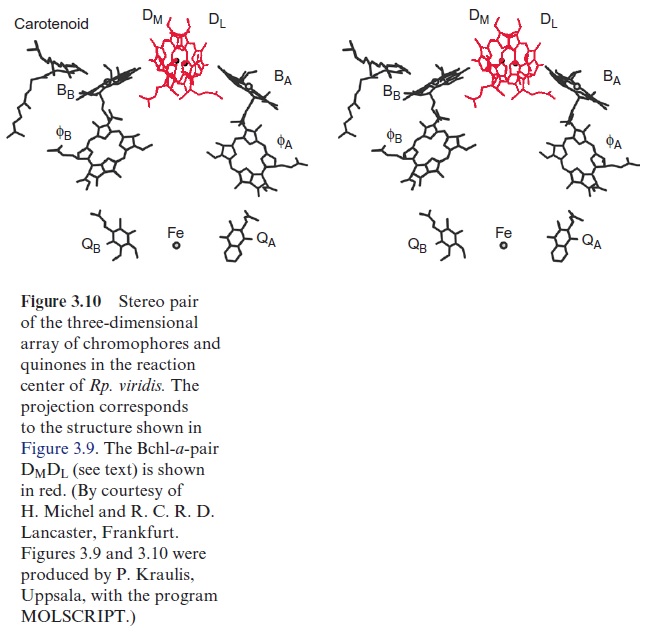
Figure 3.11 presents a scheme of the reaction center with the reaction partners arranged according to their electrochemical potential. The exci-ton of the primary reaction is provided by the antenna which then excites the chlorophyll pair. This primary excitation state has only a very short half-life time, then a charge separation occurs, and, as a result of the large potential difference, an electron is removed within picoseconds to reduce bacteriopheophytin (Bphe).
(Bchl)2 + 1 Exciton → (Bchl)2 *
(Bchl)2 * + Bphe → (Bchl)2 •+ + Bphe•-
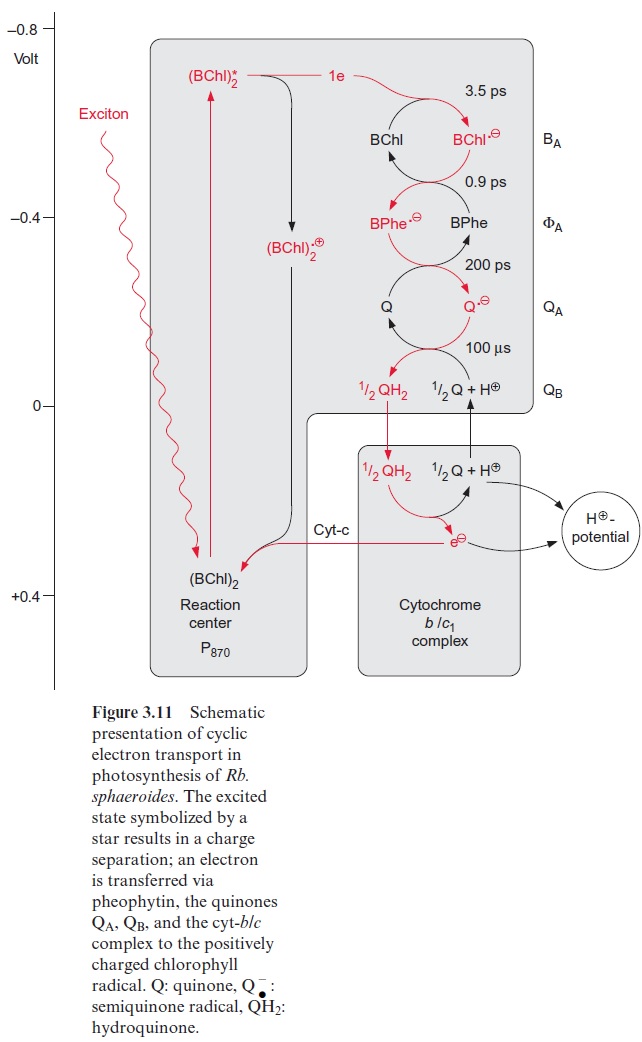
The electron is probably transferred first to the Bchl-monomer (BA) and then to the pheophytin molecule ( ФA). The second electron transfer proceeds with a half-time of 0.9 picoseconds, about four times as fast as the elec-tron transfer to BA. The pheophytin radicalhas a tendency to return to the ground state by a return of the translocated electron to the Bchl-monomer (BA). To prevent this, within 200 picoseconds a high potential difference withdraws the electron from the pheophytin radical to a quinone (QA) (Fig. 3.11). Thesemiquinone radical thus formed, in response to a further poten-tial difference, transfers its electron to the loosely bound ubiquinone QB. After a second electron transfer, first ubisemiquinone and then ubihydroqui-none are formed (Fig. 3.12). In contrast to the very labile radical intermedi-ates of the pathway, ubihydroquinone is a stable reductant. However, this stability has its price. For the formation of ubihydroquinone as a first sta-ble product from the primary excitation state of the chlorophyll, more than half of the exciton energy is dissipated as heat.
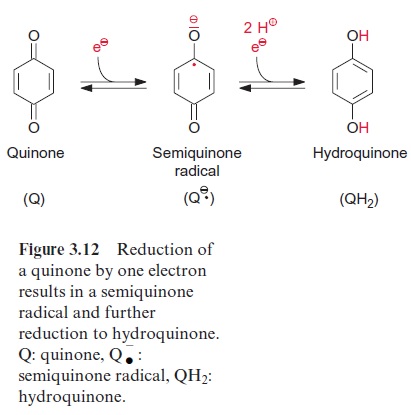
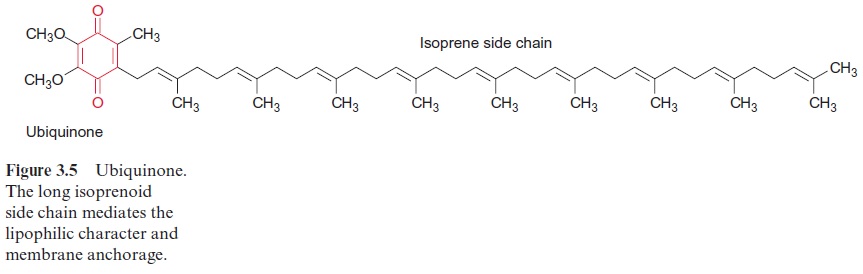
Ubiquinone (Fig. 3.5) contains a hydrophobic isoprenoid side chain, by which it is soluble in the lipid phase of the photosynthetic membrane. The same function of an isoprenoid side chain has already been discussed in the case of chlorophyll . In contrast to chlorophyll, pheophytin, and QA, which are all tightly bound to proteins, ubihydroquinone QB is only loosely associated with the reaction center and can be exchanged by another ubiquinone. Ubihydroquinone remains in the membrane phase, is able to diffuse rapidly along the membrane, and functions as a transport metabolite for reducing equivalents in the membrane phase. It feeds the electrons into the cytochrome-b/c1 complex, also located in the membrane. The electrons are then transferred back to the reaction center through the cytochrome-b/c1 complex and via cytochrome-c. Energy is conserved during this electron transport as a proton potential , which is used for ATP-synthesis.
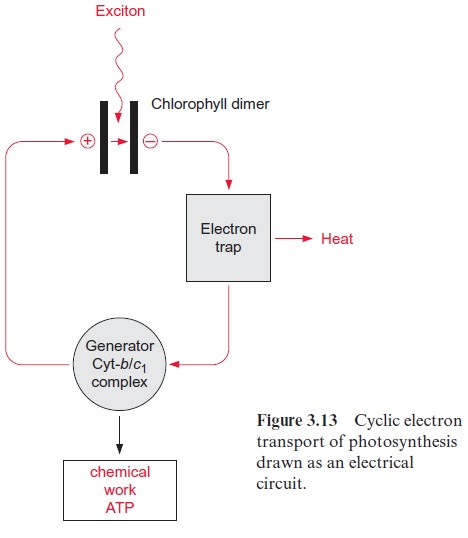
In summary, the cyclic electron transport of the purple bacteria resembles a simple electric circuit (Fig. 3.13). The two pairs of chlorophyll and pheophy-tin, between which an electron is transferred by light energy, may be regarded as the two plates of a capacitor between which a voltage is generated, driv-ing a flux of electrons, a current. Voltage drops via a resistor and a large amount of the electron energy is dissipated as heat. This resistor functions as an electron trap, and withdraws the electrons rapidly from the capacitor. A generator utilizes the remaining voltage to produce chemical energy.
Related Topics