Chapter: Plant Biochemistry: Photosynthesis is an electron transport process
In the absence of other acceptors electrons can be transferred from photosystem I to oxygen
In the absence of other acceptors electrons can be transferred from photosystem I to oxygen
When ferredoxin is very highly reduced, it is possible that electrons are transferred from PS I to oxygen to form superoxide radicals(•O2- ) (Fig. 3.35). This process is called the Mehler reaction. The superoxide radical reduces metal ions present in the cell such as Fe3+ and Cu2+ (Mn+ ):
•O2- + Mn+ → O2 + M( n-1)+
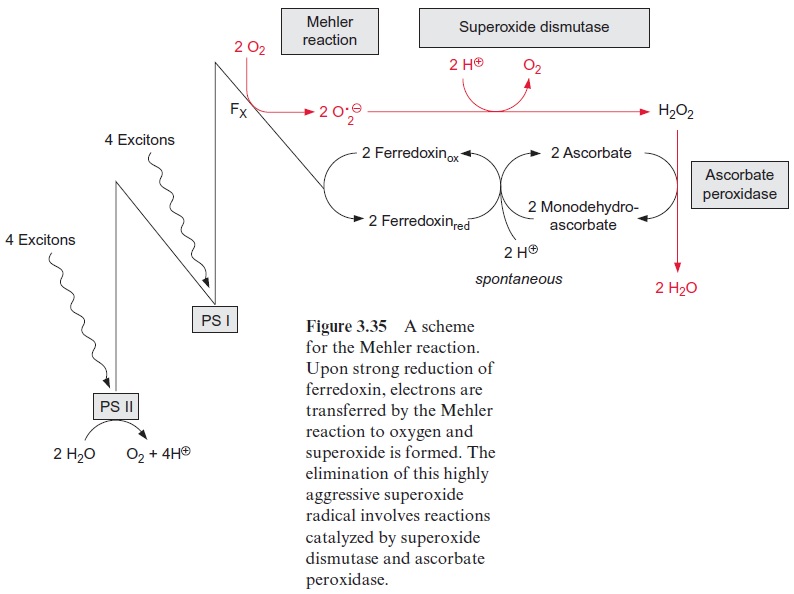
Superoxide dismutase catalyzes the dismutation of •O2- into H2O2 and O2, accompanied by the uptake of two protons:
2 • O2- + 2 H+ → O2 + H2 O2
•O2- , H2O2 and •OH are summarized as ROS (reactive oxygen species). The metal ions reduced by superoxide react with hydrogen peroxide to form hydroxyl radicals:
H2 O2 + M( n-1)+ → OH- + • OH + Mn+
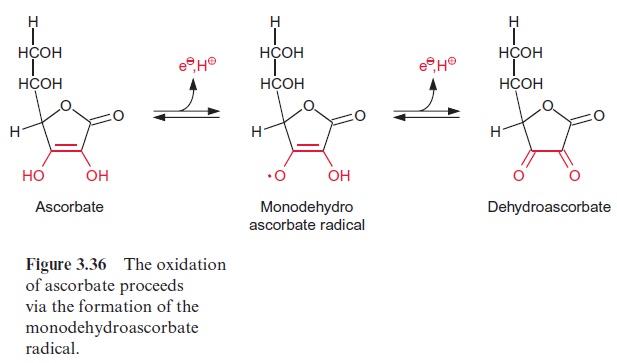
The hydroxyl radical (•OH) is a very aggressive substance and damages enzymes and lipids by oxidation. The plant cell has no protective enzymes against •OH. Therefore it is essential that a reduction of the metal ions be prevented by rapid elimination of •O2-by superoxide dismutase. But hydrogen peroxide (H2O2) also has a damaging effect on many enzymes. To prevent such damage, hydrogen peroxide is eliminated by an ascorbate peroxidase located in the thylakoid membrane. Ascorbate, an important anti-oxidant in plant cells (Fig. 3.36), is oxidized by this enzyme and converted to the radical monodehydroascorbate, which is spontaneously reconverted by photosystem I to ascorbate via reduced ferredoxin. Monodehydroascorbate can be also reduced to ascorbate by an NAD(P)H-dependent monodehy-droascorbate reductase that is present in the chloroplast stroma and the cytosol.
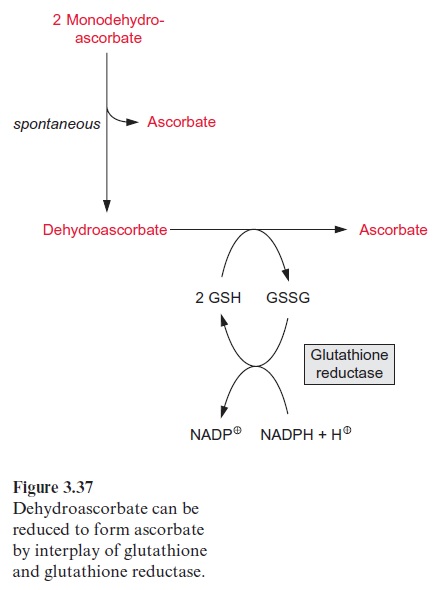
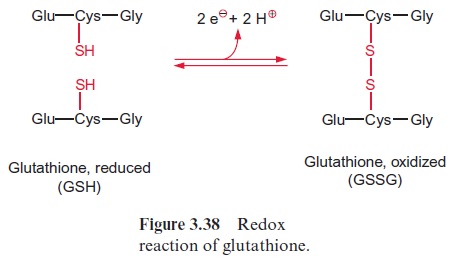
As an alternative to the preceding reaction, two molecules of mono-dehydroascorbate can dismutate to ascorbate and dehydroascorbate. Dehydroascorbate is reconverted to ascorbate by reduction with glutath-ione in a reaction catalyzed bydehydroascorbate reductase present in the stroma (Fig. 3.37). Glutathione (GSH) occurs as an antioxidant in all plant cells . It is a tripeptide composed of the amino acids glutamate, cysteine, and glycine (Fig. 3.38). Oxidation of GSH results in the forma-tion of a disulfide (GSSG) between the cysteine residues of two glutathione molecules. Reduction of GSSG is catalyzed by a glutathione reductase with NADPH as the reductant (Fig. 3.37).
The major function of the Mehler-ascorbate-peroxidase cycle is to dis-sipate excessive excitation energy of photosystem I as heat. The absorption of a total of eight excitons via PS I results in the formation of two super-oxide radicals and two molecules of reduced ferredoxin, the latter serving as a reductant for eliminating H2O2 (Fig. 3.35). The transfer of electrons to oxygen by the Mehler reaction is a reversal of the water splitting of PS II. The Mehler reaction occurs when ferredoxin is very highly reduced. The only gain of this reaction is the generation of a proton gradient from electron transport through PS II and the cyt-b6/f complex. This proton gradient can be used for the synthesis of ATP if ADP is present. But since there is usually a shortage in ADP under the conditions of the Mehler reaction, it mostly results in the formation of a high pH gradient. A feature common to the Mehler reaction and cyclic electron transport is that there is no net production of NADPH. For this reason, electron transport via the Mehler reaction has been termed pseudo-cyclic electron transport.
Yet another group of antioxidants was recently found in plants, the so-called peroxiredoxins. These proteins, comprising -SH groups as redox car-riers, have been known in the animal world for some time. Ten different peroxiredoxin genes have been identified in the model plant Arabidopsis. Peroxiredoxins, being present in chloroplasts as well as in other cell com-partments, differ from the aforementioned antioxidants glutathione and ascorbate in that they reduce a remarkably wide spectrum of peroxides, such as H2O2,alkylperoxides, and peroxinitrites. In chloroplasts, oxidized peroxiredoxins are reduced by photosynthetic electron transport of photo-system I with ferredoxin and thioredoxin as intermediates.

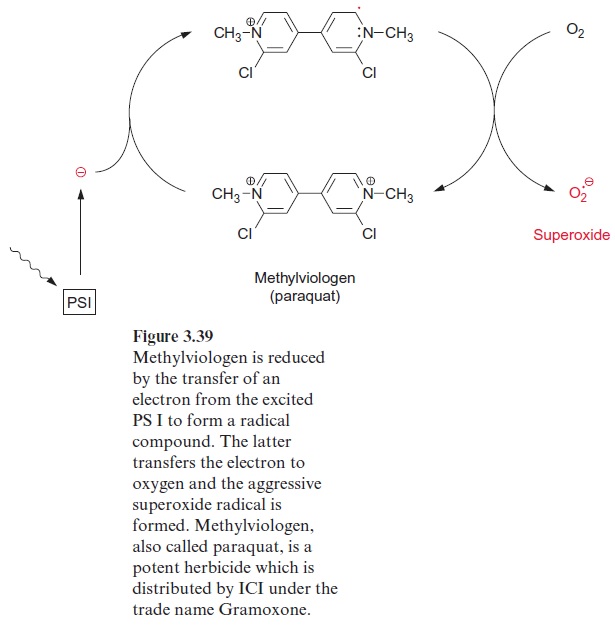
Instead of ferredoxin, PS I can also reduce methylviologen. Methylviologen, also called paraquat, is used commercially as a herbicide (Fig. 3.39). The herbicidal effect is due to the reduction of oxygen to super-oxide radicals. Additionally, paraquat competes with dehydroascorbate for the reducing equivalents provided by photosystem I. Therefore, in the pres-ence of paraquat, ascorbate is no longer regenerated from dehydroascor-bate and the ascorbate peroxidase reaction can no longer proceed. The increased production of superoxide and decreased detoxification of hydro-gen peroxide in the presence of paraquat causes severe oxidative damage to mesophyll cells, noticeable by a bleaching of the leaves. In the past, paraquat has been used to destroy marijuana fields in South America.
Related Topics