Chapter: Biochemical Pharmacology : G protein-coupled receptors
Structure and function of G protein-coupled receptors
Structure and function of G
protein-coupled receptors
G protein-coupled receptors
all belong to one structural family, which is frequently referred to as the `7-TM'
receptor family. This name refers to the 7 α-helical transmem-brane domains, which occur in
all of these molecules. Vari-ability is larger in the N-terminal and C-terminal
parts and the loops intervening between the transmembrane domains, which are
exposed to the extracellular and the cytoplasmic spaces, respectively.
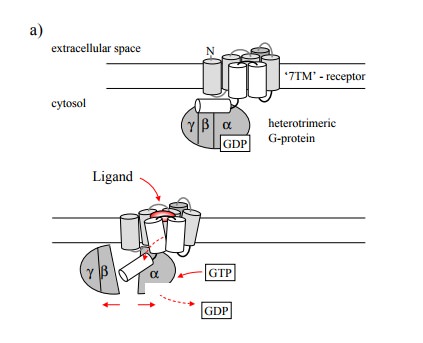
The basic mode of action of a
G protein coupled receptor and the G protein activated by it is illustrated in
Figure 8.1. Binding of the agonist to the extracellular face of the recep-tor
triggers a conformational change that is communicated to the intracellular
portion of the receptor and there is re-layed to the G protein. The latter is a
trimer, comprised of one α, β and γ subunit each. The β and γ subunits remain associated throughout the
functional cycle of the G protein. Interaction with the receptor involves all
three subunits of the G protein. When the receptor is activated, it will
trans-mit its conformational change to G protein. This will trig ger the α subunit to exchange its associated GDP molecule (a leftover from
the last round of activation) for GTP, and to dissociate from both the βγ-dimer and the receptor. It will then find and bind to its effector
molecule, which will result in either activation or inhibition of the effector.
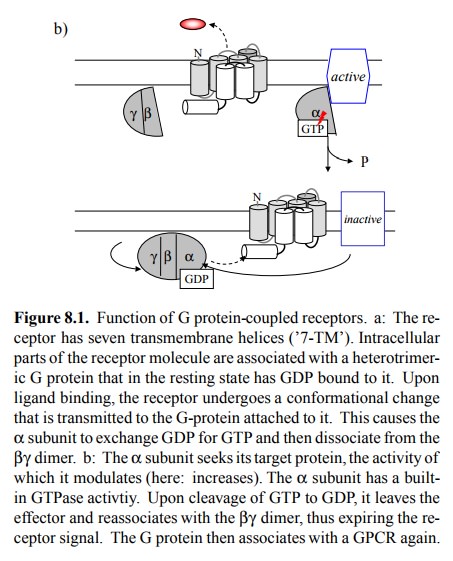
The signal is expired through
the intrinsic GTP'ase activity of the α subunit, which after some random2
time interval will cleave the GTP to GDP. This will cause the α subunit to fall back into its inactive conformation and leave the
effec-tor, which in turn will resume its previous functional state. It will
then re-associate with the βγ dimer to complete the cycle and wait for the
next round of activation event by the same or another receptor molecule.
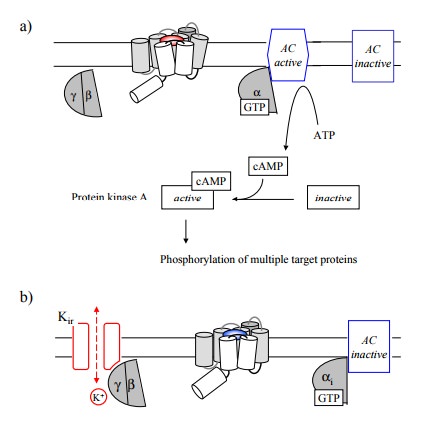
In Figure 8.1a, the effector
was shown to be activated by the G-protein. An example of this is the
stimulation of adenylate cyclase, which occurs upon stimulation e.g. of β-adrenergic receptors and is mediated by the stimulatory G protein (Gs; Figure 8.2a). Other major effector
mechanisms include:
• The inhibition of adenylate cyclase. This is mediated by the inhibitory G protein
(Gi) α subunit. Triggers for this response include
the adrenergic α2-receptor, which is responsible for presynaptic feedback inhibition
in adrenergic synapses, and the muscarinergic M2 receptor (Figure
8.2b).
• Inhibition or activation of
phosphodiesterases, in particular cGMP-specific ones. cGMP (cyclic guanosine monophosphate) is an
intracellular second messenger similar to cAMP. Besides activating a group of
protein kinases3, cGMP acts directly on several ion channels.
In the
rods and cones (visual sensory cells) of the retina, rhodopsin (a particular
type of GPCR, activated by light-induced structural change to its ligand
retinal) activates phosphodiesterase, which in turn depletes cGMP and thus
triggers the inactivation of a cGMP-dependent Na+ channel. This is not of
immediate relevance in pharmacology, but it illustrates that G protein-mediated
responses can be very fast indeed.
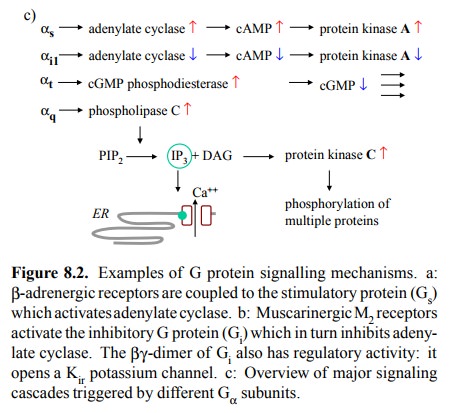
• Activation of phospholipase Cβ (Figure 8.2c). This
enzyme is associated with the
inner surface of the plasma
membrane and splits
the phospholipid
phosphatidylinositol-4,5-bisphosphate into diacylglycerol (DAG) and
inositol-1,4,5-triphosphate (IP3). Diacylglycerol is hydrophobic and remains
associated with the membrane, where it will activate protein kinase C, which in
turn will activate a broad and cell-dependent spectrum of target proteins. IP3 is
water-soluble and diffuses across the cytosol to the membrane of the en-doplasmic
reticulum, where it will activate a specific ligand-gated calcium channel.
Therefore, it will raise the cytosolic calcium level (recall that the
concentra-tion of Ca++ is higher in the ER than in the cytosol).
This is the major effector mechanism by which trans-mitters, hormones and drugs
will promote contraction in the smooth muscle (remember that calcium will
induce phosphorylation of the myosin light chain by myosin light chain kinase).
Receptors that trigger this cascade include
– The α1-adrenergic receptor, found predominantly in blood vessels, –
Various Muscarinic acetylcholine receptors, found e.g. in the intestinal smooth
muscle,
– The oxytocin receptor, found in smooth muscle
cells in the uterus, where it triggers labour.
However, the phospholipase C
cascade is not restricted to smooth muscle cells but is ubiquitous.
• Activation of phospholipase A 2,
which releases arachi-donic acid and thus initiates synthesis of prostaglandins
and related eicosanoide mediators. Again, we will see more about this in a
dedicated chapter.
• Opening or closing of ion channels. In this
way, the `metabotropic' receptors may change the membrane potential as well.
While the more numerous and
clear-cut regulatory activities of G proteins are associated with α subunits, the βγ dimers may also influence some downstream
effector. An example is the effect of the muscarinergic M2 receptor
on the activity of a potassium channel, which, like the one associated with the
sulfonylurea receptor, belongs to the `inward rectifier' class. The channel
opening effected by the βγ dimer will hyperpolarize the membrane and
reduce its responsiveness to excitation.
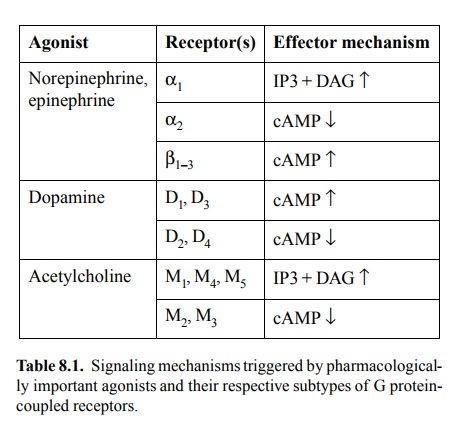
Table 8.1 lists several
transmitters, and their cognate recep-tor and signalling mechanisms. All three
of the effector mechanisms listed in table 8.1 for the adrenergic receptors
also occur among the `metabotropic' glutamate receptors.
Related Topics