Chapter: Biochemical Pharmacology : G protein-coupled receptors
Cholinesterase antagonists
Cholinesterase antagonists
The last group of agents that
affect cholinergic synaptic transmission are blockers of cholinesterase. This
enzyme has a catalytic mechanism that is analogous to that of chy-motrypsin and
related proteases. In chymotrypsin, there is a `catalytic triad', consisting of
a serine, a histidine, and an aspartic acid side chain in the active site. The
only dif-ference with cholinesterase is that a glutamate residue re-places the
aspartate – and this difference is insignificant, because glutamate and
aspartate share a carboxyl group, which is the essential feature for catalysis.
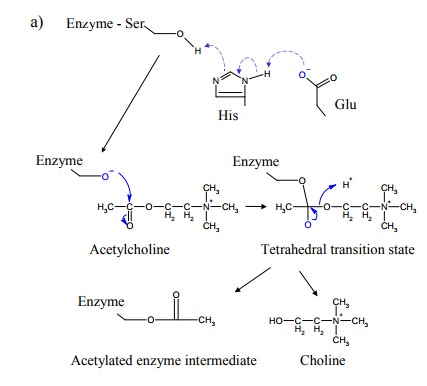
Within this catalytic triad,
the glutamate and the histidine residues cooperate to effect deprotonation of
the hydroxyl group in the serine side chain (Figure 9.15). The anionic oxygen
is a powerful nucleophile that will readily attack the carbonyl carbon of the
ester bond in acetylcholine. This will release choline and leave the acetyl
group attached (via another ester bond) to the enzyme. In order to reactivate
the enzyme, the ester bond must be cleaved again. The nucle-ophile that is
utilized in this step is a plain hydroxide anion, generated by the
deprotonation of water according to the same mechanism as seen before for the
serine.
1. Chemical groups of cholinesterase inhibitors
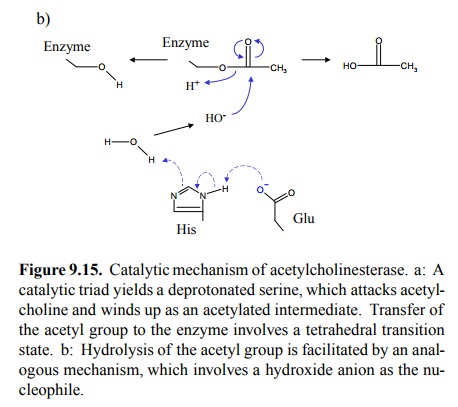
The two-step mechanism of
acetylcholinesterase provides the basis for the mode of action of most
cholinesterase in-hibitors. This is shown for the inhibitor neostigmine in
Fig-ure 9.16. Initial cleavage of the inhibitor works just fine; however, the
carbamoylated intermediate is far more stable than the acetylated one and is
only very sluggishly hydrol-ysed to regenerate the active enzyme. In fact, the
same re-action as with neostigmine occurs with carbamoylcholine, too. Thus,
carbamoylcholine is both an agonist at the re-ceptor and an inhibitor of
acetylcholinesterase. After sto-ichiometric reaction of the enzyme with the
substrate, any further reaction will be very slow, and since the drug will be
present in large excess over the enzyme, carbamoylcholine will appear resistant
to cholinesterase.
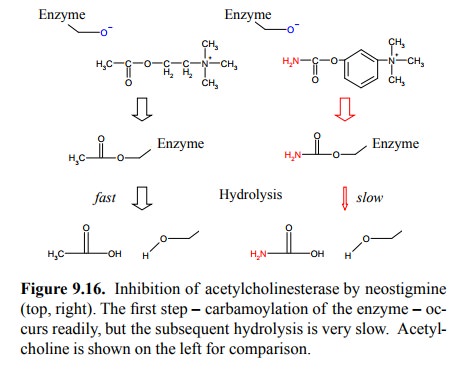
A different class of
acetylcholinesterase inhibitors are the so-called organophosphates (Figure
9.17). While acetyl-choline and the carbamoyl-based esterase inhibitors have a
trihedral structure at the site of attack (the carbonyl group) and assume a
tetrahydral structure only in the transition state of the reaction, the
organo-phosphates have a tetrahe-dral structure right from start. Since enzymes
commonly have a high binding affinity for the transition state, this explains
that organophosphate inhibitors bind very avidly to acetylcholinesterase. They
are, accordingly, extraordinarily toxic, and their small molecular size and
hydrophobic char-acter enable them to be taken up quickly by inhalation or even
across the skin.
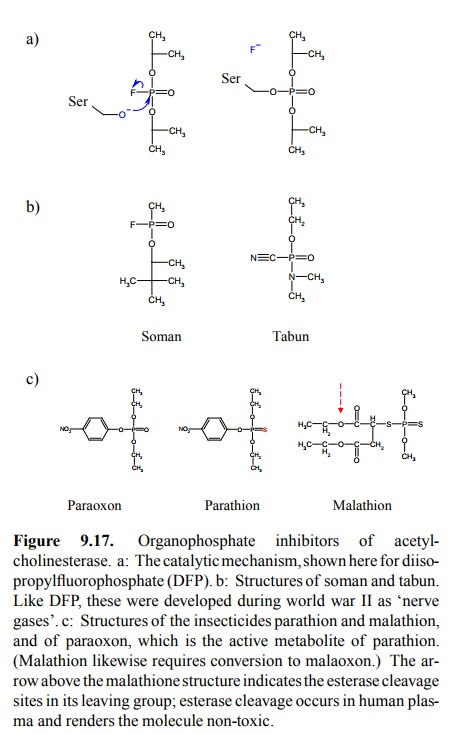
For one of the
organophosphates, diisopropylfluorophos-phate (DFP), the reaction mechanism is
outlined in Figure 9.17a. Fluorine makes an excellent leaving group and is
substituted by the active site serine. It is also found in the `nerve gas'
soman. A different leaving group (cyanide) is found in the nerve gas tabun
(Figure 9.17b).
Another factor that
contributes to the high level of toxici-ty of the organophosphates is the
stability of the phosphate ester bond formed with the enzyme: The enzyme does
not spontaneously hydrolyse itself, not even slowly as is the case with the
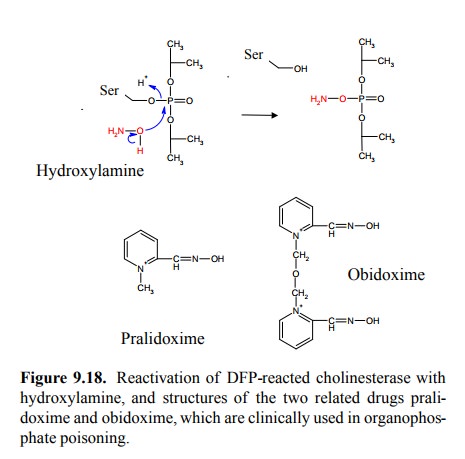
carbamates. Reactivation can be accom-plished with an extraneous nucleophile
such as hydroxy-lamine (Figure 9.18). While the latter works fine in vitro, it
is too toxic for use in vivo. However, analogs such as prali doxime and
obidoxime have been developed that steer the hydroxylamine reactive group to
the active site of acetyl-cholinesterase. This means they are effective at far lower
concentrations and can indeed be used in vivo for the treat-ment of
organophosphate poisoning.
The cholinesterase inhibitors
have multiple practical appli-cations – from medicine to warfare. Physostigmine
was actually the first practically used cholinesterase antago-nist. To describe
its initial use as `medical', however, would mean a bit of a stretch – instead,
the seed 18 containing it was used to extort confessions from
persons accused of crimes or witchcraft in Guinea. This seed used to be called
the `or-deal bean' by the local people.
2. Applications of cholinesterase inhibitors
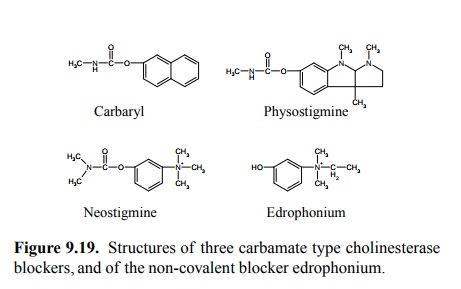
Physostigmine and other
carbamate derivatives such as neostigmine, and edrophonium (which is a
non-covalent antagonist; Figure 9.19) are preferred in medicine over organophosphates
because of their more controllable (and self-terminating) activity. One
important application is in a disease called `Myasthenia gravis
pseudoparalytica', which means `severe, quasi-paralytic muscle weakness'. This
disease is due to the pathological formation of neutral-izing (i.e.,
inactivating) antibodies against the NAR of the muscle cells. The post-synaptic
membranes will then be- come less sensitive to the acetylcholine stimulus.
Prolonga-tion of the lifetime of acetylcholine by partial blockade of
cholinesterase is used (with varying success) to compensate for this. Another
application is in glaucoma, as described above for direct cholinergic agonists.
Cholinesterase inhibitors of
both the carbamate and the organophosphate type are very important as
insecticides. Such agents include parathion (which is first converted
metabolically to paraoxon; Figure 9.17c), malathion, and carbaryl. Parathion is
very poisonous for both insects and vertebrates (including homo sapiens), and therefore has largely been abandoned. Carbaryl
binds more avidly to in-sect acetylcholinesterases and therefore has better
selectiv-ity. Malathion achieves better selectivity by the inclusion of ester
bonds within its peculiar (thiomalatediethyl ester) leaving group. Cleavage of
those (by esterases other than cholinesterase) inactivates it and appears to
proceed much more rapidly in mammals than in insects.
Organophosphates are active
not only against acetyl-cholinesterase but also serine proteases – which is
obvious-ly due to the shared catalytic mechanism. DFP is actually being used as
a protease inhibitor in biotechnology. Anoth-er inhibitor that shares its mode
of action but is less dan-gerous (because it is not volatile, and the enzyme
adducts it forms are less stable) is PMSF (phenylmethylsulfone flu-oride). You
may have encountered it in one or the other re-search lab; it is commonly added
to crude cell extracts in order to minimize enzymatic breakdown of proteins
during purification.
Related Topics