Chapter: Biochemical Pharmacology : G protein-coupled receptors
Monoamine oxidase inhibitors
Monoamine oxidase inhibitors
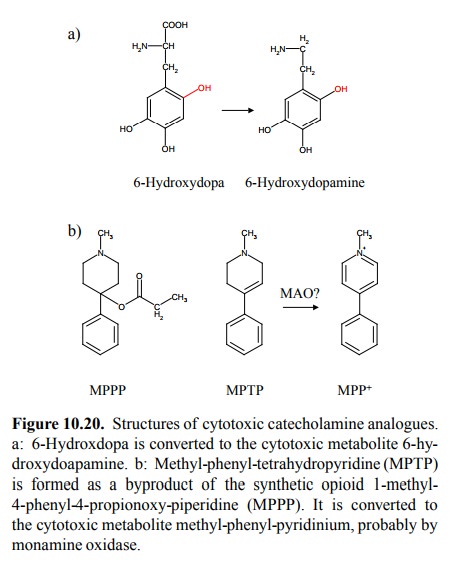
The last class of drugs we
will consider are the inhibitors of monoamine oxidase (MAO). When we compare
the re-action products in Figure 10.3 and in Figure 10.20b, re-spectively, they
look fairly different14; yet both may be accounted for by the
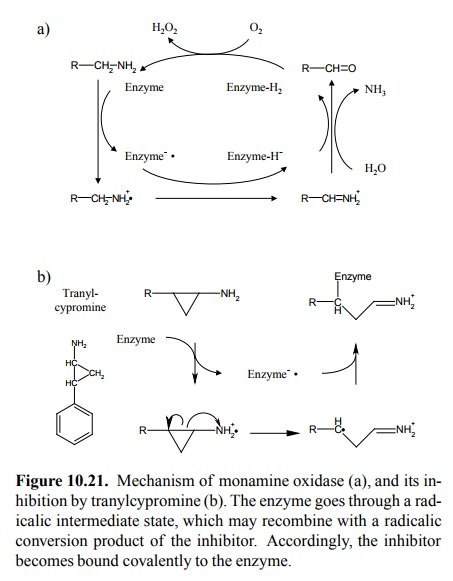
enzymatic mechanism outlined in Figure 10.21a in simplified form 15.
The enzyme reaction starts with the abstraction of an electron from the
substrate, which converts both the substrate and the enzyme to rad-icals.
Subsequently, the substrate is dehydrogenated to a Schiff Base, which in turn
is hydrolyzed to an aldehyde. Hydrogen and electrons wind up bound to the FAD
pros-thetic group of the enzyme.
The hypothetic
reaction mechanism for the inhibitor tranyl-cypromine is shown in Figure
10.21b. Abstraction of the first electron causes the (instable) cyclopropyl
ring to open, and the radical thus formed recombines with the one formed at the
enzyme to yield a covalent adduct. Because of this covalent attachment to the
enzyme, the effect of MAO inhibitors outlasts the elimination of the drug and
is only reversed by synthesis of new enzyme, which will re-quire days to weeks
after discontinuation.
MAO inhibitors will act
peripherally and may act centrally, again depending on their pharmacokinetic
properties. They have, like reserpine, been used for both antihypertensive and
antipsychotic treatment but now been superseded by more selectively acting
drugs. However, there recently has been renewed interest in the development of
MAO B-selec-tive inhibitors, since that enzyme subtype acts preferential-ly on
serotonin and in the central nervous system; some of the side effects could
thus be avoided or ameliorated. MAO B inhibitors have also been reported to
increase the lifetime of dopamine and therefore to be beneficial in Parkinson's
disease; similarly, inhibitors of COMT have more recently been introduced as a
supplement to therapy in this disease.
Hello,
wake up. So, why should MAO inhibitors have an antihypertensive effect?
Decreased degradation of catecholamines should increase the availability of
nore-pinephrine and increase rather than decrease blood pres-sure, shouldn't
it? My textbook says that it works as fol-lows (Figure 10.22): Small amounts of
tyrosine will always get decarboxylated to tyramine. Normally, tyramine is
scavenged by monoamine oxidase. However, if this path-way is blocked, tyramine
will get converted instead to oc then act
as a false transmitter, in the same way as discussed above for guanethidine and
methyl-DOPA.
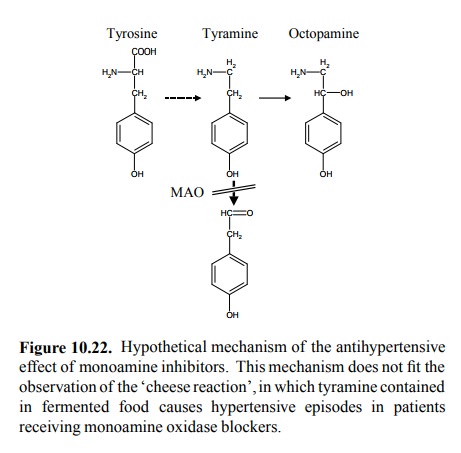
Nice
huh? But most likely wrong. The same text does not fail to mention the
so-called ‘cheese reaction’, which consists in a sudden rise of blood pressure
in patients receiving MAO inhibitors. Cheese – as well as other types of
fermented food, such as salami or summer sausage – is rich in decarboxylation
products of amino acids (amines), which are in part responsible for the
characteristic flavours. The one of interest here is indeed tyramine16.
Tyramine acts as an ‘indirect sympathomimetic’, much in the same way as
amphetamine does. It can hardly be held responsible for lowering and increasing
the blood pressure at the same time.
Related Topics