Chapter: Biochemical Pharmacology : G protein-coupled receptors
Indirect sympathomimetics
Indirect sympathomimetics
A more complex (and still somewhat contentious)
mecha-nism of action is found with a group of drugs known as `in-direct
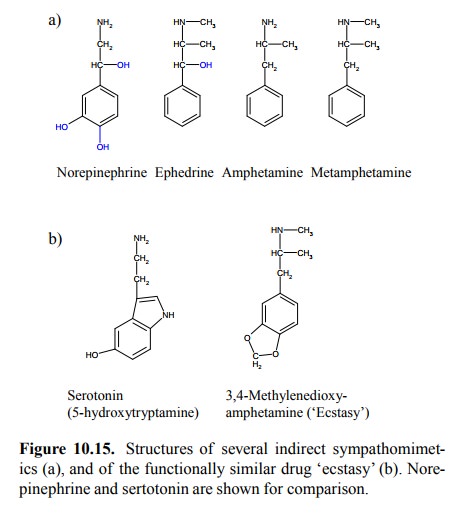
sympathomimetics'. Example structures are shown in Figure 10.15a. As you can
see, all these structures lack the phenolic –OH groups present in epinephrine.
This pre-vents their modification by catechol-O-methyltransferase and slows
down their deamination by monoamine ox-idase. It also facilitates their
crossing of the blood brain barrier, and it is mostly for their central effects
– enhanced vigilance, elation, and suppression of appetite, result-ing in
weight loss – that these drugs have been used and abused. Amphetamine and
methamphetamine preferen tially act on catecholaminergic synapses, in
particular on those containing norepinephrine. In contrast, the substance
3,4-methylenedioxyamphetamine (a.k.a. `ecstasy'; Figure 10.15b) has a stronger
effect on serotoninergic synapses. It is likewise popular as a drug of abuse.
Amphetamine
acts on both transmitter transporters – the plasmalemmal one, which is the site
of action of cocaine, and the vesicular transporter, which is targeted by
reserpine. It is imported into the cell by the plasmalemmal transporter. This
will result in inhibited reuptake of the physiological transmitter, not so much
apparently by direct competition (as is the case with cocaine) but by
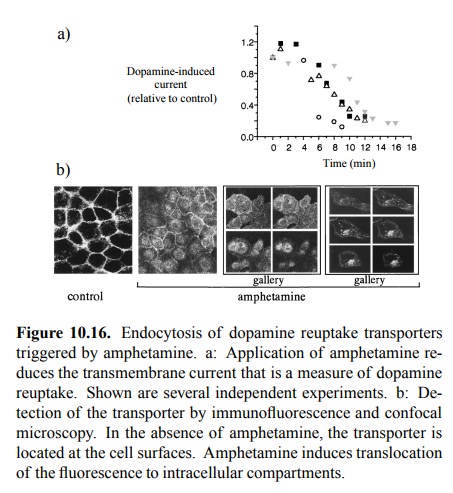
subsequent endocytosis of the receptor. This is clearly shown in Figure 10.16.
In the experiment shown, the dopamine reuptake transporter was recombinantly
expressed in cultured cells and visu-alized by immunofluorescence 9.
Initially, the fluorescence is confined to the surface of the cells expressing
the trans-porter10 (Figure 10.16b, left panel). After exposure of
the cells to amphetamine, the stain gradually disappears from the surface and is
translocated into the cell interior, indi-cating endocytosis of the transporter11.
Simultaneously, the transport capacity for dopamine drops by some 80-90%, as
measured by the current across the cell membrane induced by dopamine (Figure
10.16a). The transport can be meaplasm (cf. Figure 10.11a; note that dopamine
itself carries sured as a current, since for each molecule of dopamine taken up
there is a net transfer of 2 cations into the cytoa positive charge, too).
There is not much known about how amphetamine import triggers transporter
endocytosis.
In addition to interfering
with the reuptake of cate-cholamine transmitters, amphetamine will also release
transmitter that is stored inside vesicles. This is different from reserpine,
which only prevents uptake of more trans-mitter molecules into the vesicles but
does not cause release of those already inside. The release itself can be
demon-strated quite clearly with transmitter vesicles isolated form nerve
tissue (Figure 10.17a); its mechanism, however, is still contentious. From
several reports in the literature, I have distilled the model12
depicted in Figure 10.17b.
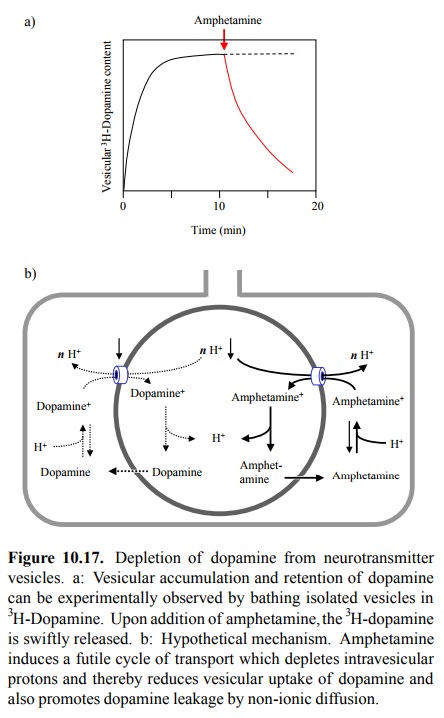
As with the plasmalemmal transporter, the ionized form of the transmitter is the one that is the substrate for active transport into the vesicle. We saw above that this transport is powered by antiport of protons from the vesicle into the cytosol. For the hypothetical mechanism of transmitter depletion to work, it is important that there be more than one proton released from the vesicle in exchange for ev-ery catecholamine molecule imported (i.e., n > 1 in Fig-ure 10.17b).
The high
proton concentration in the vesicle serves yet another purpose, which consists
in trapping the cate-cholamine in its protonated form inside the vesicle (and
thereby preventing it from slipping back across the mem-brane by non-ionic
diffusion). Amphetamine would de-rail this mechanism by again functioning as a
substrate for transport. This would lead to depletion of the protons from the
vesicle interior. Both amphetamine and the transmitter itself would thus be
present to an increased extent in their non-protonated forms and thus would
leak out of the vesi-cle by non-ionic diffusion. Amphetamine, in particular,
being less polar, would efficiently escape from the vesicle and thus by futile
cycling between cytosol and vesicle ex-haust the capacities of the proton pump
and, possibly, the transporter.
Related Topics