Chapter: Biochemical Pharmacology : G protein-coupled receptors
'Allosteric' GPCR agonists and antagonists
'Allosteric' GPCR agonists and
antagonists
While
most drugs that act as agonists or agonists of GPCR appear to bind
competitively, i.e. to the binding site of the physiological agonist, several
compounds have been report-ed to bind to other sites, allowing them to bind
simultaneously with the physiological agonist. Binding of such `al-losteric'
effectors may promote or reduce activation by the physiological agonist without
actually causing any effect in the absence of the latter. As an example, the
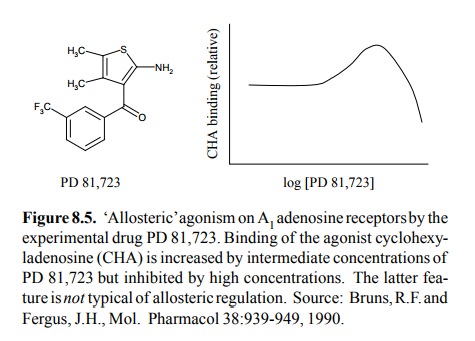
interaction of two molecules with A1 adenosine receptors5
is shown in Figure 8.5. While the `allosteric' molecule in question (PD 81,723)
does indeed enhance binding of the agonist (cyclohexyladenosine) at
intermediate concentrations, it actually inhibits it at higher concentrations,
which is not at all in keeping with the expectations for a true allosteric
effector. An alternative interpretation is that the A1 adenosine receptor is
oligomeric, and that cyclohexyladenosine and PD 81,723 bind to the regular
binding site but on separate subunits. If binding is to separate subunits were
cooperative, one would expect a pattern like the one observed here.
Related Topics