Chapter: Biochemical Pharmacology : G protein-coupled receptors
Cholinergic agonists
Cholinergic agonists
Drugs that stimulate
acetylcholine receptors are conven-tionally called `direct agonists', as
opposed to `indirect ag-onists', which are inhibitors of acetylcholinesterase
(see below). Direct cholinergic agonists are used in a variety of clinical
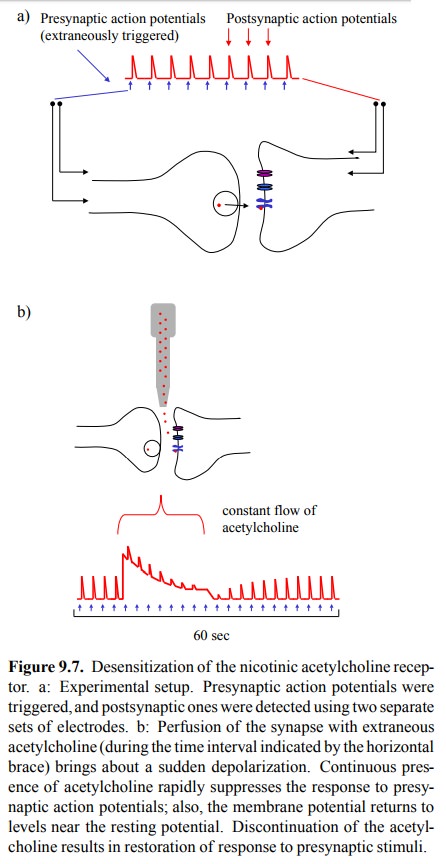
applications. Acetylcholine itself is not a very use ful drug because it gets
so rapidly hydrolysed. Just like in the experiment above (Figure 9.7), its
action in vivo sub-sides as a matter
of seconds after discontinuation. Most cholinergic agonists that are in
clinical use are partially or completely resistant to degradation by
cholinesterase and thus will remain active for extended periods of time.
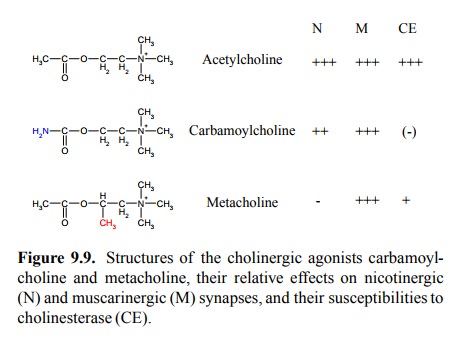
1. Muscarinic agonists
Two such agonists are shown
in Figure 9.9. In the struc-ture of carbamoylcholine, the acetyl group is
replaced by a carbamoyl group. This agonist is only very slowly de-graded by
cholinesterase. It resembles acetylcholine in being active at both muscarinic
and nicotinic synapses. However, the muscarinergic effects are stronger, and
car-bamoylcholine is being used clinically to stimulate intesti-nal and urinary
bladder motility in transient states of paral-ysis that may occur following
surgical procedures. Meta-choline retains the acetyl group; its lower susceptibility
to cholinesterase is due to a methyl group that sterically hin-ders the enzyme.
The methyl group, at the same time, ren-ders it selectively active on
muscarinic synapses. It is used for the same purposes as carbamoylcholine.
Both carbamoylcholine and metacholine are
esters and as such highly susceptible to less specific esterases in the
intes-tine and liver, so that they cannot be applied orally. Other cholinergic
agonists, however, do not possess an ester bond at all, and many of those are
indeed active after oral appli-cation.
Figure 9.10 shows two
agonists that act selectively on mus-carinic synapses: Muscarine (surprise!)
and pilocarpine. Both of these compounds have rather remote similarity with
each other or with acetylcholine. Muscarine does not have an ester bond and is
active orally. It is, however, not used therapeutically – rather, it is a
poison found in toad-stool9 and other mushrooms. It will produce the
effects – bronchial constriction, hypersalivation, intestinal cramps that can be
predicted from the distribution and functional effects of the muscarinergic
receptors that were discussed in the preceding chapter.
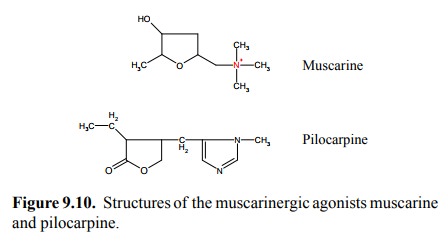
Pilocarpine, in contrast to
muscarine, is being used thera-peutically – mostly for local application to the
eye. It will cause both miosis (by action on the sphincter or constrictor
muscle of the iris) and accommodation of the eye lens for seeing close, by
acting on another (ciliary) muscle. These muscle movements will decongest a
tiny canal which is lo-cated right behind the ciliary muscle10 and
thereby facilitate the efflux of fluid from the eye. Pilocarpine is thus used
in glaucoma, a disease that is caused by pathologically in-creased pressure
within the eye.
Pilocarpine is also used
orally – not in clinical medicine, but by some natives of South America, who
for some rea-son appear to appreciate the salivation it induces and chew the
leaves of the shrub that contain it. It does not seem to cause major toxic
effects by ingestion – possibly it is again unstable in the intestine because
of its internal ester (lac-tone) bond. However, I assume that it can be taken
up to some extent across the mucous membranes.
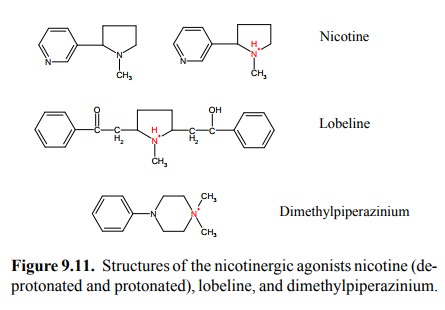
2. Nicotinic agonists
Nicotine and lobeline (Figure
9.11) are as well found in plants. Both act as agonists on nicotinic synapses,
and they share the feature of being fairly hydrophobic. The amino groups in
both of them are tertiary rather than quaternary, so that both are capable of
non-ionic diffusion, enabling them to pass across membrane barriers. With
nicotine, this is apparent in its effect on the central nervous system (along
with that upon the peripheral autonomic ganglia). In addi-tion, nicotine is
also used in plasters and chewing gums11 by those who want to quit
smoking, which is evidence that it can cross mucous membranes and even the skin
with ease. In contrast to nicotine, dimethylpiperazinium has a quater-nary
amino group. It is therefore permanently ionic and does not easily cross the
blood-brain barrier. It does not have any clinical applications I'm aware of,
but it is used in research with experimental animals for selective stimula-tion
of the autonomic ganglia in the absence of simultane-ous central effects.
The peripheral autonomic
effects of nicotine and similar agents are somewhat irregular, due to the fact
that both sympathetic and parasympathetic ganglia are stimulated, which will
result in partially antagonistic actions. How-ever, in general, the effects on
the intestine will be main-ly parasympathetic, which can be nicely observed in
small boys smoking their first cigars. Conversely, the effect on the
cardiovascular system is predominantly sympathetic, which may be related to the
fact that not only the ganglia but also the adrenal glands are being
stimulated. With the dosages that are required for the autonomic effects, the
functional changes at the motor endplate (= neuromuscular synapse) are
irrelevant; at very high concentrations, depo-larizing blockade (see later) may
be induced.
Related Topics